当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel TRβ selective agonists sustained by ADME-toxicity analysis.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.112006 Massimiliano Runfola 1 , Simona Sestito 1 , Lorenza Bellusci 2 , Valeria La Pietra 3 , Vincenzo Maria D'Amore 3 , Marta Anna Kowalik 4 , Grazia Chiellini 2 , Sheraz Gul 5 , Andrea Perra 4 , Amedeo Columbano 4 , Luciana Marinelli 3 , Ettore Novellino 3 , Simona Rapposelli 6
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.112006 Massimiliano Runfola 1 , Simona Sestito 1 , Lorenza Bellusci 2 , Valeria La Pietra 3 , Vincenzo Maria D'Amore 3 , Marta Anna Kowalik 4 , Grazia Chiellini 2 , Sheraz Gul 5 , Andrea Perra 4 , Amedeo Columbano 4 , Luciana Marinelli 3 , Ettore Novellino 3 , Simona Rapposelli 6
Affiliation
|
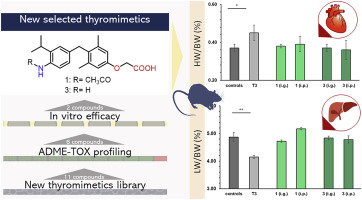
Although triiodothyronine (T3) induces several beneficial effects on lipid metabolism, its use is hampered by toxic side-effects, such as tachycardia, arrhythmia, heart failure, bone and muscle catabolism and mood disturbances. Since the α isoform of thyroid hormone receptors (TRs) is the main cause of T3-related harmful effects, several efforts have been made to develop selective agonists of the β isoform that could induce some beneficial effects (i.e. lowering triglyceride and cholesterol levels reducing obesity and improving metabolic syndrome), while overcoming most of the adverse T3-dependent side effects. Herein, we describe the drug discovery process sustained by ADME-Toxicity analysis that led us to identify novel agonists with selectivity for the isoform TRβ and an acceptable off-target and absorption, distribution metabolism, excretion and toxicity (ADME-Tox) profile. Within the small series of compounds synthesized, derivatives 1 and 3, emerge from this analysis as "potentially safe" to be engaged in preclinical studies. In in vitro investigation proved that both compounds were able to reduce lipid accumulation in HepG2 and promote lipolysis with comparable effects to those elicited by T3, used as reference drug. Moreover, a preliminary in vivo study confirmed the apparent lack of toxicity, thus suggesting compounds 1 and 3 as new potential TRβ-selective thyromimetics.
中文翻译:

通过ADME毒性分析获得的新型TRβ选择性激动剂的设计,合成和生物学评估。
尽管三碘甲状腺素(T3)对脂质代谢具有多种有益作用,但其使用受到毒性副反应的阻碍,例如心动过速,心律不齐,心力衰竭,骨骼和肌肉分解代谢以及情绪障碍。由于甲状腺激素受体(TRs)的α同工型是T3相关有害作用的主要原因,因此已经做出了一些努力来开发选择性诱导β同工型的激动剂,这些激动剂可以诱导某些有益作用(例如降低甘油三酸酯和胆固醇水平,降低肥胖症)并改善了代谢综合征),同时克服了大多数不良的T3依赖性副作用。在这里,我们描述了由ADME-毒性分析支持的药物发现过程,该过程使我们确定了对同种型TRβ具有选择性,可接受的脱靶和吸收,分布代谢,排泄和毒性(ADME-Tox)图。在合成的小系列化合物中,衍生物1和3从该分析中显示出可从事临床前研究的“潜在安全性”。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解作用,其效果与用作参考药物的T3所产生的效果相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解作用,其效果与用作参考药物的T3所产生的效果相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解,其作用与用作参考药物的T3所产生的作用相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。
更新日期:2019-12-23
中文翻译:

通过ADME毒性分析获得的新型TRβ选择性激动剂的设计,合成和生物学评估。
尽管三碘甲状腺素(T3)对脂质代谢具有多种有益作用,但其使用受到毒性副反应的阻碍,例如心动过速,心律不齐,心力衰竭,骨骼和肌肉分解代谢以及情绪障碍。由于甲状腺激素受体(TRs)的α同工型是T3相关有害作用的主要原因,因此已经做出了一些努力来开发选择性诱导β同工型的激动剂,这些激动剂可以诱导某些有益作用(例如降低甘油三酸酯和胆固醇水平,降低肥胖症)并改善了代谢综合征),同时克服了大多数不良的T3依赖性副作用。在这里,我们描述了由ADME-毒性分析支持的药物发现过程,该过程使我们确定了对同种型TRβ具有选择性,可接受的脱靶和吸收,分布代谢,排泄和毒性(ADME-Tox)图。在合成的小系列化合物中,衍生物1和3从该分析中显示出可从事临床前研究的“潜在安全性”。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解作用,其效果与用作参考药物的T3所产生的效果相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解作用,其效果与用作参考药物的T3所产生的效果相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。体外研究证明,这两种化合物均能够减少HepG2中的脂质蓄积并促进脂解,其作用与用作参考药物的T3所产生的作用相当。此外,一项初步的体内研究证实明显缺乏毒性,因此表明化合物1和3是新的潜在的TRβ-选择性胸腺肽药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号