当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Minor chemical modifications of the aminosteroid derivative RM-581 lead to major impact on its anticancer activity, metabolic stability and aqueous solubility.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.111990 René Maltais 1 , Martin Perreault 2 , Jenny Roy 1 , Donald Poirier 2
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.111990 René Maltais 1 , Martin Perreault 2 , Jenny Roy 1 , Donald Poirier 2
Affiliation
|
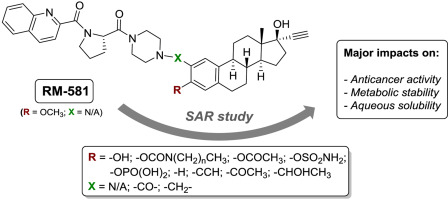
The aminosteroid (AM) RM-581 is built around a mestranol backbone and has recently emerged as this family's lead candidate, showing in vitro and in vivo potency over different types of cancer, including high fatality pancreatic cancer. To extend the structure-activity relationships (SAR) to other estrane analogs, we synthesized a focused series of RM-581 derivatives at position C3 or C2 of its steroidal core. These new AM derivatives were first tested on a large selection of prostate, breast, pancreatic and ovarian cancer cell lines. The impact of these modifications on metabolic stability (human liver microsomes) was also measured. A SAR study revealed a fine regulation of anticancer activity related to the nature of the substituent. Indeed, the addition of potential prodrug groups like acetate, sulfamate or phosphate (compounds 8, 9 and 10) at C3 of the phenolic counterpart provided better antiproliferative activities than RM-581 in breast and pancreatic cancer cell types while maintaining activity in other cancer cell lines. Also, the phosphate group was highly beneficial on water solubility. However, the bulkier carbamate prodrugs 6 (N,N-dimethyl) and 7 (N,N-diethyl) were less active. Otherwise, carbon homologation (CH2) at C2 (compound 33) was beneficial to metabolic stability and, in the meantime, this AM conserved the same anticancer activity as RM-581. However, the replacement of the hydroxy or methoxy at C3 by a hydrogen or an acetyl (compound 17 or 21b) was detrimental for anticancer activity, pointing to a crucial molecular interaction of the aromatic oxygen atom at this position. Overall, this work provided a better knowledge of the structural requirements to maintain RM-581's anticancer activity, and also identified minor structural modifications to increase both metabolic stability and water solubility, three important parameters of pharmacological development.
中文翻译:

氨基类固醇衍生物RM-581的微小化学修饰对其抗癌活性,代谢稳定性和水溶性具有重大影响。
氨基类固醇(AM)RM-581围绕甲羟色胺骨架构建,最近作为该家族的主要候选药物出现,显示出对包括高致死性胰腺癌在内的不同类型癌症的体外和体内效力。为了将结构-活性关系(SAR)扩展到其他雌激素类似物,我们在其甾体核心的C3或C2位置合成了一系列聚焦的RM-581衍生物。这些新的AM衍生物首先在多种选择的前列腺癌,乳腺癌,胰腺癌和卵巢癌细胞系上进行了测试。还测量了这些修饰对代谢稳定性(人肝微粒体)的影响。SAR研究表明,与取代基的性质有关的抗癌活性有很好的调节作用。确实,添加了潜在的前药基团,例如乙酸盐,氨基磺酸盐或磷酸盐(化合物8,9和10)在乳腺癌和胰腺癌细胞类型中,酚类对应物的C3处提供的抗增殖活性比RM-581好,同时保持了其他癌细胞系中的活性。同样,磷酸酯基团对水溶性是非常有益的。然而,较大的氨基甲酸酯前药6(N,N-二甲基)和7(N,N-二乙基)的活性较低。否则,C2(化合物33)处的碳同源性(CH2)有利于代谢稳定性,与此同时,该AM保留与RM-581相同的抗癌活性。但是,用氢或乙酰基(化合物17或21b)取代C3处的羟基或甲氧基对抗癌活性有害,这表明该位置上的芳族氧原子至关重要的分子相互作用。全面的,
更新日期:2019-12-23
中文翻译:

氨基类固醇衍生物RM-581的微小化学修饰对其抗癌活性,代谢稳定性和水溶性具有重大影响。
氨基类固醇(AM)RM-581围绕甲羟色胺骨架构建,最近作为该家族的主要候选药物出现,显示出对包括高致死性胰腺癌在内的不同类型癌症的体外和体内效力。为了将结构-活性关系(SAR)扩展到其他雌激素类似物,我们在其甾体核心的C3或C2位置合成了一系列聚焦的RM-581衍生物。这些新的AM衍生物首先在多种选择的前列腺癌,乳腺癌,胰腺癌和卵巢癌细胞系上进行了测试。还测量了这些修饰对代谢稳定性(人肝微粒体)的影响。SAR研究表明,与取代基的性质有关的抗癌活性有很好的调节作用。确实,添加了潜在的前药基团,例如乙酸盐,氨基磺酸盐或磷酸盐(化合物8,9和10)在乳腺癌和胰腺癌细胞类型中,酚类对应物的C3处提供的抗增殖活性比RM-581好,同时保持了其他癌细胞系中的活性。同样,磷酸酯基团对水溶性是非常有益的。然而,较大的氨基甲酸酯前药6(N,N-二甲基)和7(N,N-二乙基)的活性较低。否则,C2(化合物33)处的碳同源性(CH2)有利于代谢稳定性,与此同时,该AM保留与RM-581相同的抗癌活性。但是,用氢或乙酰基(化合物17或21b)取代C3处的羟基或甲氧基对抗癌活性有害,这表明该位置上的芳族氧原子至关重要的分子相互作用。全面的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号