当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and optimization of novel pyridines as highly potent and selective glycogen synthase kinase 3 inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bmcl.2019.126930 Savithri Ramurthy 1 , Keith B Pfister 2 , Rustum S Boyce 3 , Sean P Brown 4 , Abran Q Costales 5 , Manoj C Desai 6 , Eric Fang 7 , Barry H Levine 8 , Simon C Ng 9 , John M Nuss 10 , David B Ring 11 , Cynthia M Shafer 12 , Wei Shu 2 , Sharadha Subramanian 13 , Allan S Wagman 14 , Haixia Wang 15 , Dirksen E Bussiere 2
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bmcl.2019.126930 Savithri Ramurthy 1 , Keith B Pfister 2 , Rustum S Boyce 3 , Sean P Brown 4 , Abran Q Costales 5 , Manoj C Desai 6 , Eric Fang 7 , Barry H Levine 8 , Simon C Ng 9 , John M Nuss 10 , David B Ring 11 , Cynthia M Shafer 12 , Wei Shu 2 , Sharadha Subramanian 13 , Allan S Wagman 14 , Haixia Wang 15 , Dirksen E Bussiere 2
Affiliation
|
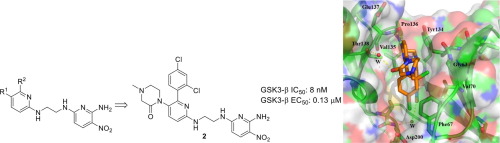
Glycogen synthase kinase-3 plays an essential role in multiple biochemical pathways in the cell, particularly in regards to energy regulation. As such, Glycogen synthase kinase-3 is an attractive target for pharmacological intervention in a variety of disease states, particularly non-insulin dependent diabetes mellitus. However, due to homology with other crucial kinases, such as the cyclin-dependent protein kinase CDC2, developing compounds that are both potent and selective is challenging. A novel series of derivatives of 5-nitro-N2-(2-(pyridine-2ylamino)ethyl)pyridine-2,6-diamine were synthesized and have been shown to potently inhibit glycogen synthase kinase-3 (GSK3). Potency in the low nanomolar range was obtained along with remarkable selectivity. The compounds activate glycogen synthase in insulin receptor-expressing CHO-IR cells and in primary rat hepatocytes, and have acceptable pharmacokinetics and pharmacodynamics to allow for oral dosing. The X-ray co-crystal structure of human GSK3-β in complex with compound 2 is reported and provides insights into the structural determinants of the series responsible for its potency and selectivity.
中文翻译:

发现和优化新型吡啶作为高效和选择性糖原合酶激酶3抑制剂。
糖原合酶激酶3在细胞的多个生化途径中起着至关重要的作用,特别是在能量调节方面。这样,糖原合酶激酶-3是在多种疾病状态,特别是非胰岛素依赖性糖尿病中的药理干预的有吸引力的靶标。但是,由于与其他关键激酶(如细胞周期蛋白依赖性蛋白激酶CDC2)的同源性,开发既有效又具有选择性的化合物具有挑战性。合成了一系列新的5-硝基-N2-(2-(吡啶-2-基氨基)乙基)吡啶-2,6-二胺衍生物,并已显示出可有效抑制糖原合酶激酶-3(GSK3)。在低纳摩尔范围内获得了出色的选择性。该化合物在表达胰岛素受体的CHO-IR细胞和原代大鼠肝细胞中激活糖原合酶,并具有可接受的药代动力学和药效学,可以口服给药。报告了人GSK3-β与化合物2形成复合物的X射线共晶体结构,该结构提供了有关其效力和选择性的系列结构决定因素的见解。
更新日期:2019-12-23
中文翻译:

发现和优化新型吡啶作为高效和选择性糖原合酶激酶3抑制剂。
糖原合酶激酶3在细胞的多个生化途径中起着至关重要的作用,特别是在能量调节方面。这样,糖原合酶激酶-3是在多种疾病状态,特别是非胰岛素依赖性糖尿病中的药理干预的有吸引力的靶标。但是,由于与其他关键激酶(如细胞周期蛋白依赖性蛋白激酶CDC2)的同源性,开发既有效又具有选择性的化合物具有挑战性。合成了一系列新的5-硝基-N2-(2-(吡啶-2-基氨基)乙基)吡啶-2,6-二胺衍生物,并已显示出可有效抑制糖原合酶激酶-3(GSK3)。在低纳摩尔范围内获得了出色的选择性。该化合物在表达胰岛素受体的CHO-IR细胞和原代大鼠肝细胞中激活糖原合酶,并具有可接受的药代动力学和药效学,可以口服给药。报告了人GSK3-β与化合物2形成复合物的X射线共晶体结构,该结构提供了有关其效力和选择性的系列结构决定因素的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号