当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient synthesis and cell migration inhibitory effect of substituted benzamidothiazolylpyrazole-capped AWD*I-NH2.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bmcl.2019.126914 Mian Yang 1 , Jun Chen 1 , Wancai Peng 1 , Qiqi Li 1 , Hui Shao 1 , Guanping Tang 1 , Tong-Cun Zhang 2 , Yoshikazhu Takada 3 , Long Ye 1 , Xing-Hua Liao 2
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bmcl.2019.126914 Mian Yang 1 , Jun Chen 1 , Wancai Peng 1 , Qiqi Li 1 , Hui Shao 1 , Guanping Tang 1 , Tong-Cun Zhang 2 , Yoshikazhu Takada 3 , Long Ye 1 , Xing-Hua Liao 2
Affiliation
|
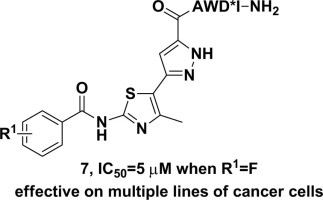
Substituted (2-benzamidothiazol-5-yl)pyrazole-capped AWD*I-NH2 were synthesized and their antimigration activity was studied. The improved efficiency and scalability of the analog synthesis was achieved via a late-stage diversification of the benzoyl group and a convergent route in which the bisazole capping agents and off-resin peptide AWD*I-NH2 were prepared in parallel and coupled together in solution at the last step. Bioassay results indicate that all the peptidomimetics can significantly inhibit the migration of breast cancer cells MDA-MB-231 but possess no apparent cytotoxicity. In general, the antimigration potency of the peptidomimetics is correlated to the electron-withdrawing capacity of the substituents on the terminal phenyl ring. The inhibitory effect shows dose-dependent and holds also against lung and cervical cancer cells. The level of f-actin was reduced dramatically in cells treated with the inhibitor, suggesting that the migration inhibitory effect is related to the disruption of cell locomotive protrusions.
中文翻译:

取代苯甲酰胺基偶氮基吡唑封端的AWD * I-NH2的高效合成和细胞迁移抑制作用。
合成了取代的(2-苯甲并噻唑-5-基)吡唑封端的AWD * I-NH2,并研究了它们的抗迁移活性。模拟合成的效率和可扩展性的提高是通过苯甲酰基的后期多样化和一条收敛途径实现的,在该途径中,平行制备双唑封端剂和脱树脂肽AWD * I-NH2,并在溶液中偶联在一起在最后一步。生物测定结果表明,所有拟肽都可以显着抑制乳腺癌细胞MDA-MB-231的迁移,但没有明显的细胞毒性。通常,拟肽的抗迁移能力与末端苯环上取代基的吸电子能力相关。抑制作用显示出剂量依赖性,并且对肺癌和宫颈癌细胞也具有抑制作用。
更新日期:2019-12-23
中文翻译:

取代苯甲酰胺基偶氮基吡唑封端的AWD * I-NH2的高效合成和细胞迁移抑制作用。
合成了取代的(2-苯甲并噻唑-5-基)吡唑封端的AWD * I-NH2,并研究了它们的抗迁移活性。模拟合成的效率和可扩展性的提高是通过苯甲酰基的后期多样化和一条收敛途径实现的,在该途径中,平行制备双唑封端剂和脱树脂肽AWD * I-NH2,并在溶液中偶联在一起在最后一步。生物测定结果表明,所有拟肽都可以显着抑制乳腺癌细胞MDA-MB-231的迁移,但没有明显的细胞毒性。通常,拟肽的抗迁移能力与末端苯环上取代基的吸电子能力相关。抑制作用显示出剂量依赖性,并且对肺癌和宫颈癌细胞也具有抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号