当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent routes in organic synthesis: carbohydrates, amino acids, alkaloids and terpenes.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02419k Carmen Nájera 1 , Francisco Foubelo 2 , José M Sansano 2 , Miguel Yus 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02419k Carmen Nájera 1 , Francisco Foubelo 2 , José M Sansano 2 , Miguel Yus 1
Affiliation
|
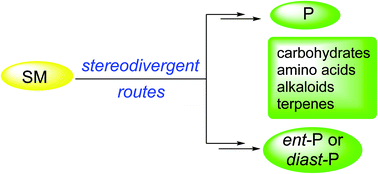
The natural occurrence of enantiomers and diastereomers is often encountered. In addition, the synthesis of these stereoisomers is important for structure determination and for the study of structure-activity relationships. Stereodivergent routes simplify the access to these molecules starting from a common material. This review is focused on the synthesis of carbohydrates, amino acids, alkaloids and terpenes using this efficient strategy. In the case of carbohydrates, such as monosaccharides, carbasugars, aminosugars and azasugars, carbohydrates are usually employed as common starting materials. As a very common strategy, configurations of hydroxy groups are inverted by SN2 methods playing with protection and deprotection processes. For the synthesis of acyclic α-AAs, diastereoselective methods using mainly Garner's aldehyde have been described. Diastereodivergent routes allowed the synthesis of β-hydroxy- and β-amino-α-amino acids, as well as of β- and γ-amino acids. Heterocyclic and cyclic amino phosphonic acids were synthesized using diastereodivergent routes. Alkaloids containing five- and six-membered saturated azaheterocycles needed multistep stereodivergent routes and other alkaloids, such as enantiomers of balanol, vincamine, anatoxin and codeine, and diastereomeric isochaetominines C and galanthamines. In the terpene field, sesquiterpenes β-santalene, α-curcumene and α-cuparenone and the diterpene scopadulcic acid A have been synthesized using enantiodivergent routes.
中文翻译:

有机合成中的立体发散途径:碳水化合物,氨基酸,生物碱和萜烯。
通常遇到对映异构体和非对映异构体的自然存在。另外,这些立体异构体的合成对于结构确定和结构-活性关系的研究很重要。立体发散途径简化了从常见材料开始接近这些分子的过程。这篇综述着重于使用这种有效策略合成碳水化合物,氨基酸,生物碱和萜烯。在碳水化合物的情况下,例如单糖,Carcarbugars,氨基糖和氮杂糖,通常将碳水化合物用作常见的起始原料。作为一种非常常见的策略,通过SN2方法将羟基的构型反转,同时进行保护和脱保护过程。对于合成无环α-AA,主要使用Garner's的非对映选择性方法 已经描述了乙醛。非对映异构途径允许合成β-羟基-和β-氨基-α-氨基酸,以及β-和γ-氨基酸。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。
更新日期:2020-02-19
中文翻译:

有机合成中的立体发散途径:碳水化合物,氨基酸,生物碱和萜烯。
通常遇到对映异构体和非对映异构体的自然存在。另外,这些立体异构体的合成对于结构确定和结构-活性关系的研究很重要。立体发散途径简化了从常见材料开始接近这些分子的过程。这篇综述着重于使用这种有效策略合成碳水化合物,氨基酸,生物碱和萜烯。在碳水化合物的情况下,例如单糖,Carcarbugars,氨基糖和氮杂糖,通常将碳水化合物用作常见的起始原料。作为一种非常常见的策略,通过SN2方法将羟基的构型反转,同时进行保护和脱保护过程。对于合成无环α-AA,主要使用Garner's的非对映选择性方法 已经描述了乙醛。非对映异构途径允许合成β-羟基-和β-氨基-α-氨基酸,以及β-和γ-氨基酸。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。使用非对映异构路线合成杂环和环状氨基膦酸。含有五元和六元饱和氮杂杂环的生物碱需要多步立体发散途径,还需要其他生物碱,如巴拉诺尔,长春胺,抗毒素和可待因的对映异构体,以及非对映异构的异二十二碳三烯酸丙啶和加兰他敏。在萜烯领域中,使用对映异构路线合成了倍半萜烯β-檀香烯,α-姜黄烯和α-cuparenone以及二萜烯scopadulicicA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号