当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct evidence of the key role of UV-formed peroxide species in photocatalytic gas-solid oxidation in air on anatase TiO2 particles.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp04728j Irina Rudol'fovna Subbotina 1 , Denis Valer'evich Barsukov
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp04728j Irina Rudol'fovna Subbotina 1 , Denis Valer'evich Barsukov
Affiliation
|
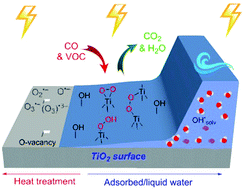
IR spectroscopy was applied for the in situ investigations of surface intermediates formed on the surface of TiO2 (anatase) and ZnO under UV light illumination in air and their reactivity in the elimination of simple pollutant molecules. UV-irradiation of TiO2 (anatase) in air leads to the generation of peroxo-species with the peaks at 852 and 912 cm-1, but the bands of O2˙-ads were not detected. This is, to our knowledge, the first direct in situ IR spectroscopic detection of O2 photosorption intermediates in moist air. The formation of peroxo-species in these conditions is specific for TiO2 (anatase), whereas on ZnO the predominant species under UV light illumination in air are O2˙-ads and H2O2, desorbing into gas phase. Adsorbed water and surface hydroxyl groups contribute to the formation and stabilization of peroxo-species on TiO2 anatase during UV illumination in an oxygen atmosphere. If UV-irradiation is carried out in the environment of moist argon instead of moist air, the peroxo-species on TiO2 anatase are formed from water in a negligible quantity. Peroxo-species formed after O2 photoadsorption on TiO2 anatase in moist air have band positions similar to peroxo-species formed after photodecomposition of H2O2 (with accompanying color change of this sample from yellow to white). Direct experimental IR-spectroscopic evidence of peroxo-species reactivity as oxidative intermediates on TiO2 (anatase) in CO and ethanol vapor photooxidative processes is firstly obtained. These results confirm our early conclusion that peroxo-species formed under UV-irradiation in O2 on the hydrated surface of TiO2 (anatase) can be responsible for the surprising extreme dependence of the CO photooxidation rate on the adsorbed water coverage with the maximum at ∼0.5 ML. The ZnO sample was not active in the photooxidation of these molecules in air. It is concluded that UV formed peroxo-species are important diamagnetic oxidative intermediates in heterogeneous photochemical gas-solid oxidation processes on TiO2 (anatase).
中文翻译:

在锐钛矿型TiO2颗粒上,紫外线形成的过氧化物在空气中的光催化气固氧化中的关键作用的直接证据。
红外光谱用于在空气中紫外线照射下在TiO2(锐钛矿)和ZnO表面形成的表面中间体及其在消除简单污染物分子中的反应性的原位研究。空气中TiO2(锐钛矿)的紫外线照射导致生成过氧物种,其峰值分别在852和912 cm-1,但未检测到O2 + -ads谱带。据我们所知,这是首次直接原位红外光谱检测潮湿空气中的O2光吸收中间体。在这些条件下,过氧物种的形成是特定于TiO2(锐钛矿)的,而在ZnO上,紫外线在空气中的主要种类是O2 + -ads和H2O2,它们解吸成气相。在氧气气氛中进行紫外线照射期间,吸附的水和表面羟基有助于TiO2锐钛矿上过氧物种的形成和稳定。如果在潮湿的氩气环境中而不是潮湿的空气中进行UV辐射,则TiO2锐钛矿上的过氧物种是由水形成的,数量可忽略不计。O2在湿空气中的TiO2锐钛矿上光吸附O2后形成的过氧物种具有与H2O2光分解后形成的过氧物种相似的能带位置(该样品的颜色从黄色变为白色)。首先获得直接的红外光谱实验证据,证明过氧物种作为CO和乙醇蒸气光氧化过程中TiO2(锐钛矿)上的氧化中间体的反应性。这些结果证实了我们的早期结论,即在O2的紫外线照射下,TiO2水合表面上形成的过氧物种(锐钛矿)可能是CO光氧化速率对吸附水覆盖率的令人惊讶的极端依赖性,其最大值约为0.5。 ML。ZnO样品在空气中这些分子的光氧化中没有活性。可以得出结论,紫外线形成的过氧物种是TiO2(锐钛矿)的非均相光化学气固氧化过程中的重要反磁性氧化中间体。
更新日期:2020-01-15
中文翻译:

在锐钛矿型TiO2颗粒上,紫外线形成的过氧化物在空气中的光催化气固氧化中的关键作用的直接证据。
红外光谱用于在空气中紫外线照射下在TiO2(锐钛矿)和ZnO表面形成的表面中间体及其在消除简单污染物分子中的反应性的原位研究。空气中TiO2(锐钛矿)的紫外线照射导致生成过氧物种,其峰值分别在852和912 cm-1,但未检测到O2 + -ads谱带。据我们所知,这是首次直接原位红外光谱检测潮湿空气中的O2光吸收中间体。在这些条件下,过氧物种的形成是特定于TiO2(锐钛矿)的,而在ZnO上,紫外线在空气中的主要种类是O2 + -ads和H2O2,它们解吸成气相。在氧气气氛中进行紫外线照射期间,吸附的水和表面羟基有助于TiO2锐钛矿上过氧物种的形成和稳定。如果在潮湿的氩气环境中而不是潮湿的空气中进行UV辐射,则TiO2锐钛矿上的过氧物种是由水形成的,数量可忽略不计。O2在湿空气中的TiO2锐钛矿上光吸附O2后形成的过氧物种具有与H2O2光分解后形成的过氧物种相似的能带位置(该样品的颜色从黄色变为白色)。首先获得直接的红外光谱实验证据,证明过氧物种作为CO和乙醇蒸气光氧化过程中TiO2(锐钛矿)上的氧化中间体的反应性。这些结果证实了我们的早期结论,即在O2的紫外线照射下,TiO2水合表面上形成的过氧物种(锐钛矿)可能是CO光氧化速率对吸附水覆盖率的令人惊讶的极端依赖性,其最大值约为0.5。 ML。ZnO样品在空气中这些分子的光氧化中没有活性。可以得出结论,紫外线形成的过氧物种是TiO2(锐钛矿)的非均相光化学气固氧化过程中的重要反磁性氧化中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号