Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma.
Nature Medicine ( IF 58.7 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41591-019-0694-x Sangeeta Goswami 1 , Thomas Walle 2, 3 , Andrew E Cornish 4, 5 , Sreyashi Basu 6 , Swetha Anandhan 1 , Irina Fernandez 6 , Luis Vence 6 , Jorge Blando 6 , Hao Zhao 6 , Shalini Singh Yadav 6 , Martina Ott 7 , Ling Y Kong 7 , Amy B Heimberger 7 , John de Groot 8 , Boris Sepesi 9 , Michael Overman 10 , Scott Kopetz 10 , James P Allison 6, 11 , Dana Pe'er 4 , Padmanee Sharma 1, 6, 11
Nature Medicine ( IF 58.7 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41591-019-0694-x Sangeeta Goswami 1 , Thomas Walle 2, 3 , Andrew E Cornish 4, 5 , Sreyashi Basu 6 , Swetha Anandhan 1 , Irina Fernandez 6 , Luis Vence 6 , Jorge Blando 6 , Hao Zhao 6 , Shalini Singh Yadav 6 , Martina Ott 7 , Ling Y Kong 7 , Amy B Heimberger 7 , John de Groot 8 , Boris Sepesi 9 , Michael Overman 10 , Scott Kopetz 10 , James P Allison 6, 11 , Dana Pe'er 4 , Padmanee Sharma 1, 6, 11
Affiliation
|
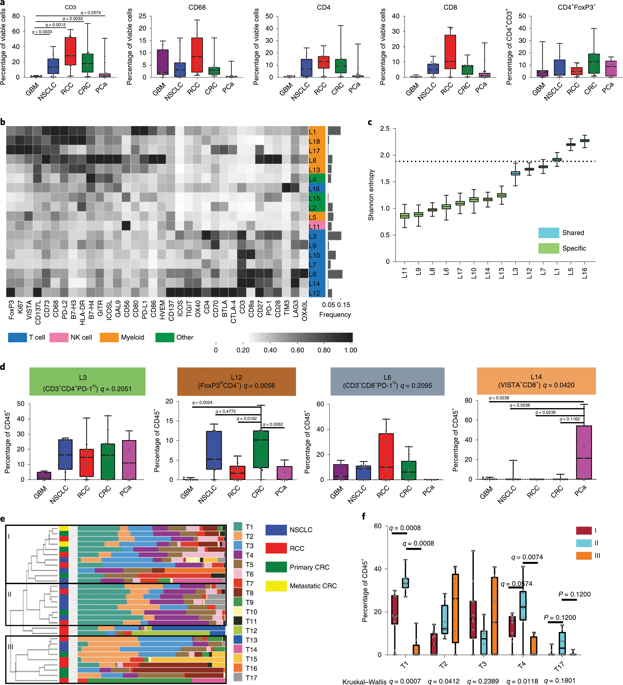
Immune checkpoint therapy with anti-CTLA-4 and anti-PD-1/PD-L1 has revolutionized the treatment of many solid tumors. However, the clinical efficacy of immune checkpoint therapy is limited to a subset of patients with specific tumor types1,2. Multiple clinical trials with combinatorial immune checkpoint strategies are ongoing; however, the mechanistic rationale for tumor-specific targeting of immune checkpoints is elusive. To garner an insight into tumor-specific immunomodulatory targets, we analyzed 94 patients representing five different cancer types, including those that respond relatively well to immune checkpoint therapy and those that do not, such as glioblastoma multiforme, prostate cancer and colorectal cancer. Through mass cytometry and single-cell RNA sequencing, we identified a unique population of CD73hi macrophages in glioblastoma multiforme that persists after anti-PD-1 treatment. To test if targeting CD73 would be important for a successful combination strategy in glioblastoma multiforme, we performed reverse translational studies using CD73-/- mice. We found that the absence of CD73 improved survival in a murine model of glioblastoma multiforme treated with anti-CTLA-4 and anti-PD-1. Our data identified CD73 as a specific immunotherapeutic target to improve antitumor immune responses to immune checkpoint therapy in glioblastoma multiforme and demonstrate that comprehensive human and reverse translational studies can be used for rational design of combinatorial immune checkpoint strategies.
中文翻译:

人类肿瘤的免疫分析将 CD73 鉴定为胶质母细胞瘤中的组合靶标。
抗 CTLA-4 和抗 PD-1/PD-L1 的免疫检查点疗法已经彻底改变了许多实体瘤的治疗。然而,免疫检查点疗法的临床疗效仅限于具有特定肿瘤类型 1、2 的患者子集。多项联合免疫检查点策略的临床试验正在进行中;然而,免疫检查点的肿瘤特异性靶向的机制原理是难以捉摸的。为了深入了解肿瘤特异性免疫调节靶点,我们分析了代表五种不同癌症类型的 94 名患者,包括对免疫检查点治疗反应相对较好的患者和对多形性胶质母细胞瘤、前列腺癌和结直肠癌反应不佳的患者。通过质谱流式细胞仪和单细胞 RNA 测序,我们在多形性胶质母细胞瘤中发现了一个独特的 CD73hi 巨噬细胞群,在抗 PD-1 治疗后仍然存在。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为一种特异性免疫治疗靶点,可改善多形性胶质母细胞瘤对免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人体和反向转化研究可用于合理设计组合免疫检查点策略。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为一种特异性免疫治疗靶点,可改善多形性胶质母细胞瘤对免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人体和反向转化研究可用于合理设计组合免疫检查点策略。
更新日期:2019-12-23
中文翻译:

人类肿瘤的免疫分析将 CD73 鉴定为胶质母细胞瘤中的组合靶标。
抗 CTLA-4 和抗 PD-1/PD-L1 的免疫检查点疗法已经彻底改变了许多实体瘤的治疗。然而,免疫检查点疗法的临床疗效仅限于具有特定肿瘤类型 1、2 的患者子集。多项联合免疫检查点策略的临床试验正在进行中;然而,免疫检查点的肿瘤特异性靶向的机制原理是难以捉摸的。为了深入了解肿瘤特异性免疫调节靶点,我们分析了代表五种不同癌症类型的 94 名患者,包括对免疫检查点治疗反应相对较好的患者和对多形性胶质母细胞瘤、前列腺癌和结直肠癌反应不佳的患者。通过质谱流式细胞仪和单细胞 RNA 测序,我们在多形性胶质母细胞瘤中发现了一个独特的 CD73hi 巨噬细胞群,在抗 PD-1 治疗后仍然存在。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。为了测试靶向 CD73 对于多形性胶质母细胞瘤的成功组合策略是否重要,我们使用 CD73-/- 小鼠进行了反向翻译研究。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为特定的免疫治疗靶点,以改善对多形性胶质母细胞瘤免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人类和反向转化研究可用于合理设计组合免疫检查点策略。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为一种特异性免疫治疗靶点,可改善多形性胶质母细胞瘤对免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人体和反向转化研究可用于合理设计组合免疫检查点策略。我们发现,在用抗 CTLA-4 和抗 PD-1 治疗的多形性胶质母细胞瘤小鼠模型中,CD73 的缺失提高了存活率。我们的数据将 CD73 确定为一种特异性免疫治疗靶点,可改善多形性胶质母细胞瘤对免疫检查点治疗的抗肿瘤免疫反应,并证明全面的人体和反向转化研究可用于合理设计组合免疫检查点策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号