当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of PTP1B activation through disruption of redox-complex formation.
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41589-019-0433-0 Avinash D Londhe 1 , Alexandre Bergeron 2, 3 , Stephanie M Curley 1 , Fuming Zhang 4 , Keith D Rivera 5 , Akaash Kannan 1 , Gérald Coulis 1, 3 , Syed H M Rizvi 1 , Seung Jun Kim 6 , Darryl J Pappin 5 , Nicholas K Tonks 5 , Robert J Linhardt 4 , Benoit Boivin 1, 2, 3, 5
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41589-019-0433-0 Avinash D Londhe 1 , Alexandre Bergeron 2, 3 , Stephanie M Curley 1 , Fuming Zhang 4 , Keith D Rivera 5 , Akaash Kannan 1 , Gérald Coulis 1, 3 , Syed H M Rizvi 1 , Seung Jun Kim 6 , Darryl J Pappin 5 , Nicholas K Tonks 5 , Robert J Linhardt 4 , Benoit Boivin 1, 2, 3, 5
Affiliation
|
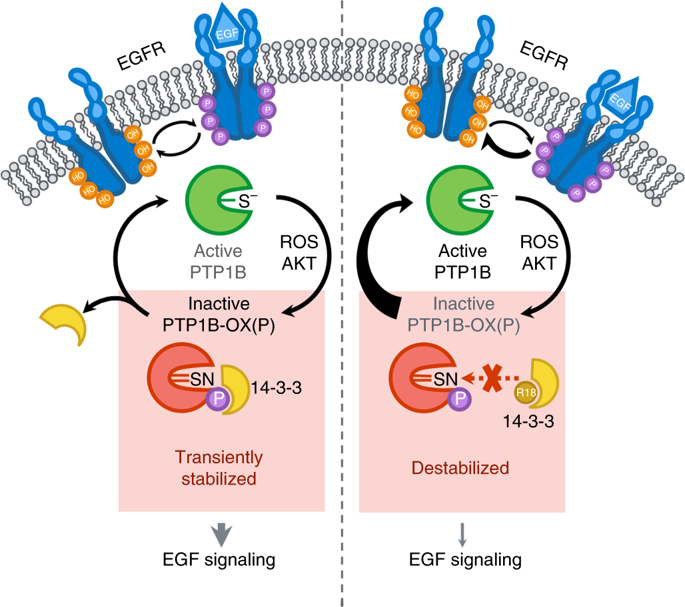
We have identified a molecular interaction between the reversibly oxidized form of protein tyrosine phosphatase 1B (PTP1B) and 14-3-3ζ that regulates PTP1B activity. Destabilizing the transient interaction between 14-3-3ζ and PTP1B prevented PTP1B inactivation by reactive oxygen species and decreased epidermal growth factor receptor phosphorylation. Our data suggest that destabilizing the interaction between 14-3-3ζ and the reversibly oxidized and inactive form of PTP1B may establish a path to PTP1B activation in cells.
中文翻译:

通过破坏氧化还原复合物的形成来调节PTP1B的活化。
我们已经确定了蛋白质酪氨酸磷酸酶1B(PTP1B)和14-3-3ζ调节PTP1B活性的可逆氧化形式之间的分子相互作用。使14-3-3ζ与PTP1B之间的瞬时相互作用不稳定,可防止PTP1B被活性氧灭活并减少表皮生长因子受体的磷酸化。我们的数据表明,破坏14-3-3ζ与PTP1B的可逆氧化和无活性形式之间的相互作用可能会为细胞中PTP1B的活化建立一条途径。
更新日期:2019-12-23
中文翻译:

通过破坏氧化还原复合物的形成来调节PTP1B的活化。
我们已经确定了蛋白质酪氨酸磷酸酶1B(PTP1B)和14-3-3ζ调节PTP1B活性的可逆氧化形式之间的分子相互作用。使14-3-3ζ与PTP1B之间的瞬时相互作用不稳定,可防止PTP1B被活性氧灭活并减少表皮生长因子受体的磷酸化。我们的数据表明,破坏14-3-3ζ与PTP1B的可逆氧化和无活性形式之间的相互作用可能会为细胞中PTP1B的活化建立一条途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号