当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic investigation of the coalescence susceptibility of pea protein-stabilised emulsions: Effect of protein oxidation level
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.foodhyd.2019.105610 Emma B.A. Hinderink , Wael Kaade , Leonard Sagis , Karin Schroën , Claire C. Berton-Carabin
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.foodhyd.2019.105610 Emma B.A. Hinderink , Wael Kaade , Leonard Sagis , Karin Schroën , Claire C. Berton-Carabin
|
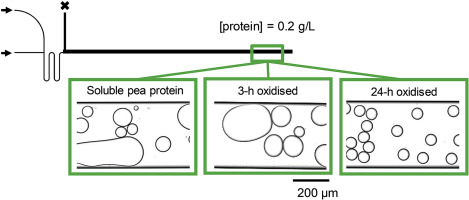
Abstract Proteins are used to stabilise oil-in-water (O/W) emulsions, and plant proteins are gaining interest as functional ingredients due to their higher sustainability potential compared to e.g., dairy proteins. However, their emulsifying properties are not that well understood, and depend on how their production process affects their physicochemical status. In the present work, we use the soluble fraction of commercial pea protein isolate to stabilise O/W emulsion droplets formed in a microfluidic device, and record coalescence stability after droplet formation (11–173 ms) for different protein concentrations (0.1–1 g/L). For the shortest adsorption times (11–65 ms) droplets were unstable, whereas for longer adsorption times differences in coalescence stability could be charted. Metal-catalysed oxidation of pea proteins performed for up to 24-h, prior to emulsion formation and analysis, increased the coalescence stability of the droplets, compared to fresh pea proteins. This may be explained by oxidation-induced protein fragmentation, leading to low molecular weight products. The Langmuir-Blodgett films looked highly heterogeneous for films prepared with fresh or mildly oxidised (3-h) proteins, and was more homogenous for 24-h oxidised proteins. This could be the cause for the observed differences in emulsion coalescence stability, structurally heterogeneous films being more prone to rupture. From this work, it is clear that the emulsifying properties of pea are strongly dependent on their chemical status, and associated structural properties at the molecular and supramolecular levels. The present microfluidic device is an efficient tool to capture such effects, at time scales that are relevant to industrial emulsification.
中文翻译:

豌豆蛋白稳定乳液聚结敏感性的微流体研究:蛋白质氧化水平的影响
摘要 蛋白质用于稳定水包油 (O/W) 乳液,并且植物蛋白质作为功能性成分越来越受到关注,因为与例如乳蛋白相比,它们具有更高的可持续性潜力。然而,它们的乳化特性还不是很清楚,取决于它们的生产过程如何影响它们的物理化学状态。在目前的工作中,我们使用商业豌豆分离蛋白的可溶性部分来稳定微流体装置中形成的 O/W 乳液液滴,并记录液滴形成后(11-173 ms)对于不同蛋白质浓度(0.1-1 g)的聚结稳定性/L)。对于最短的吸附时间(11-65 毫秒),液滴是不稳定的,而对于较长的吸附时间,可以绘制出聚结稳定性的差异。与新鲜豌豆蛋白相比,在乳液形成和分析之前进行长达 24 小时的豌豆蛋白的金属催化氧化增加了液滴的聚结稳定性。这可能是由于氧化诱导的蛋白质断裂,导致低分子量产物。Langmuir-Blodgett 薄膜对于用新鲜或轻度氧化 (3-h) 蛋白质制备的薄膜看起来高度异质,而对于 24-h 氧化蛋白质则更均匀。这可能是观察到的乳液聚结稳定性差异的原因,结构异质膜更容易破裂。从这项工作中,很明显豌豆的乳化特性在很大程度上取决于它们的化学状态,以及分子和超分子水平的相关结构特性。
更新日期:2020-05-01
中文翻译:

豌豆蛋白稳定乳液聚结敏感性的微流体研究:蛋白质氧化水平的影响
摘要 蛋白质用于稳定水包油 (O/W) 乳液,并且植物蛋白质作为功能性成分越来越受到关注,因为与例如乳蛋白相比,它们具有更高的可持续性潜力。然而,它们的乳化特性还不是很清楚,取决于它们的生产过程如何影响它们的物理化学状态。在目前的工作中,我们使用商业豌豆分离蛋白的可溶性部分来稳定微流体装置中形成的 O/W 乳液液滴,并记录液滴形成后(11-173 ms)对于不同蛋白质浓度(0.1-1 g)的聚结稳定性/L)。对于最短的吸附时间(11-65 毫秒),液滴是不稳定的,而对于较长的吸附时间,可以绘制出聚结稳定性的差异。与新鲜豌豆蛋白相比,在乳液形成和分析之前进行长达 24 小时的豌豆蛋白的金属催化氧化增加了液滴的聚结稳定性。这可能是由于氧化诱导的蛋白质断裂,导致低分子量产物。Langmuir-Blodgett 薄膜对于用新鲜或轻度氧化 (3-h) 蛋白质制备的薄膜看起来高度异质,而对于 24-h 氧化蛋白质则更均匀。这可能是观察到的乳液聚结稳定性差异的原因,结构异质膜更容易破裂。从这项工作中,很明显豌豆的乳化特性在很大程度上取决于它们的化学状态,以及分子和超分子水平的相关结构特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号