当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Reactivity of Re(III) and Re(V) Fischer Carbenes
Organometallics ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.organomet.9b00600 Caleb A. Brown 1 , Cassandra P. Lilly 1 , Nikola S. Lambic 1 , Roger D. Sommer 1 , Elon A. Ison 1
Organometallics ( IF 2.5 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.organomet.9b00600 Caleb A. Brown 1 , Cassandra P. Lilly 1 , Nikola S. Lambic 1 , Roger D. Sommer 1 , Elon A. Ison 1
Affiliation
|
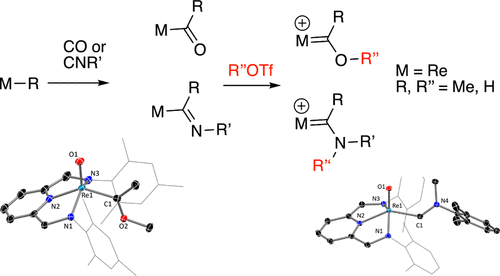
Direct insertion of CO and isocyanides, RNC, into Re–R bonds results in high-oxidation-state acyl and iminoacyl complexes that can be treated with an electrophile to generate rare examples of rhenium(III) and oxorhenium(V) Fischer carbenes. Experimental and computational studies suggest that, as expected, the carbene ligands are electrophilic at carbon. Further, an interesting correlation was observed between the 13C NMR chemical shift and the natural charge at the carbene carbon, which suggests that the electrophilicity of the ligand can be tuned, with the substituent attached to the carbene carbon having the most influence.
中文翻译:

Re(III)和Re(V)费希尔卡宾酯的合成与反应性
将CO和异氰酸酯RNC直接插入Re-R键会导致高氧化态的酰基和亚氨基酰基络合物,可以用亲电试剂处理,生成稀有的examples(III)和(V)费希尔碳酸盐。实验和计算研究表明,与预期的一样,卡宾配体在碳原子上是亲电的。此外,在13 C NMR化学位移与卡宾碳上的自然电荷之间观察到有趣的相关性,这表明可以调节配体的亲电性,其中连接至卡宾碳的取代基影响最大。
更新日期:2019-12-23
中文翻译:

Re(III)和Re(V)费希尔卡宾酯的合成与反应性
将CO和异氰酸酯RNC直接插入Re-R键会导致高氧化态的酰基和亚氨基酰基络合物,可以用亲电试剂处理,生成稀有的examples(III)和(V)费希尔碳酸盐。实验和计算研究表明,与预期的一样,卡宾配体在碳原子上是亲电的。此外,在13 C NMR化学位移与卡宾碳上的自然电荷之间观察到有趣的相关性,这表明可以调节配体的亲电性,其中连接至卡宾碳的取代基影响最大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号