当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of Co-Mingled 99Tc and Cr Removal during Mineral Transformation of Ferrous Hydroxide
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsearthspacechem.9b00277 Sarah A. Saslow 1 , Carolyn I. Pearce 1 , Mark E. Bowden 2 , Wayne W. Lukens 3 , Dong-Sang Kim 1 , Albert A. Kruger 4 , Wooyong Um 1, 5, 6
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsearthspacechem.9b00277 Sarah A. Saslow 1 , Carolyn I. Pearce 1 , Mark E. Bowden 2 , Wayne W. Lukens 3 , Dong-Sang Kim 1 , Albert A. Kruger 4 , Wooyong Um 1, 5, 6
Affiliation
|
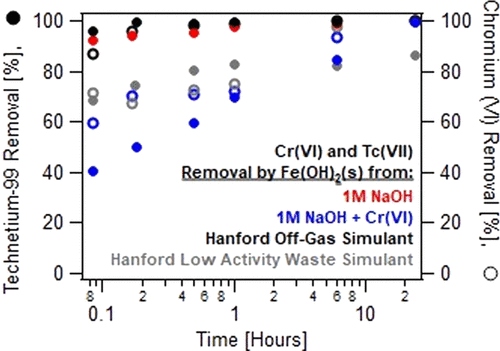
Four simulated waste streams relevant to the vitrification of Hanford nuclear waste were studied to evaluate the removal kinetics of technetium-99 (Tc) and co-mingled Cr(VI) during treatment with solid ferrous hydroxide (Fe(OH)2(s)). Simulants treated with Fe(OH)2(s) were reacted for 24 h and sub-sampled periodically to monitor Tc and Cr removal. Solution sample analysis during the reaction was coupled with solid phase characterization, for example, X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD), to establish influence of the solid phase product on Tc and Cr removal rates. Based on these results, the majority of Tc and Cr removal occurs within the first 5 min of simulant contact with Fe(OH)2(s). However, the order in which Tc and Cr are completely removed from each simulant depends on the simulant chemistry, the preferred reduction pathway, and the solid phase product, for example, magnetite (Fe3O4) versus goethite (α-FeOOH). Low pH and low Cr concentrations favor rapid Tc removal, with XRD and XAS confirming that Fe3O4 readily incorporates reduced Tc(IV) into its structure. High pH, high Cr concentrations, and the presence of other co-mingled constituents favor heterogeneous removal of Tc early in the reaction (<1 h) with removal rates often faster than those determined for Cr. At reaction times of >1 h, Tc removal slows as homogeneous removal of Cr begins to dominate, concurrent with an increase in the formation of FeOOH as the solid phase reaction product. These results suggest that for complex, high pH waste streams, Tc removal within the first hour after Fe(OH)2(s) treatment is necessary for complete Tc reduction and improved mineral immobilization.
中文翻译:

氢氧化亚铁矿物转化过程中99 Tc和Cr共混去除的动力学
研究了与Hanford核废料的玻璃化有关的四种模拟废料流,以评估在固体氢氧化亚铁(Fe(OH)2(s))处理过程中-99(Tc)和共混的Cr(VI)的去除动力学。。用Fe(OH)2(s)处理的模拟物反应24小时,并定期进行二次采样以监测Tc和Cr的去除。反应过程中的溶液样品分析与固相表征(例如X射线吸收光谱(XAS)和X射线衍射(XRD))相结合,以建立固相产物对Tc和Cr去除速率的影响。根据这些结果,大部分Tc和Cr的去除发生在与Fe(OH)2的模拟接触的前5分钟内(s)。但是,从每种模拟物中完全去除Tc和Cr的顺序取决于模拟物的化学性质,优选的还原途径和固相产物,例如磁铁矿(Fe 3 O 4)与针铁矿(α-FeOOH)。低pH和低Cr浓度有利于Tc的快速去除,XRD和XAS证实了Fe 3 O 4的存在。容易将还原的Tc(IV)纳入其结构。高pH,高Cr浓度和其他共混成分的存在有利于在反应早期(<1 h)异质去除Tc,且去除速率通常快于对Cr的去除速率。在> 1 h的反应时间,由于Cr的均匀去除开始占主导地位,因此Tc的去除速度变慢,同时固相反应产物FeOOH的形成增加。这些结果表明,对于复杂的高pH废物流,Fe(OH)2(s)处理后第一个小时内的Tc去除对于完全降低Tc和改善矿物质固定性是必要的。
更新日期:2020-01-07
中文翻译:

氢氧化亚铁矿物转化过程中99 Tc和Cr共混去除的动力学
研究了与Hanford核废料的玻璃化有关的四种模拟废料流,以评估在固体氢氧化亚铁(Fe(OH)2(s))处理过程中-99(Tc)和共混的Cr(VI)的去除动力学。。用Fe(OH)2(s)处理的模拟物反应24小时,并定期进行二次采样以监测Tc和Cr的去除。反应过程中的溶液样品分析与固相表征(例如X射线吸收光谱(XAS)和X射线衍射(XRD))相结合,以建立固相产物对Tc和Cr去除速率的影响。根据这些结果,大部分Tc和Cr的去除发生在与Fe(OH)2的模拟接触的前5分钟内(s)。但是,从每种模拟物中完全去除Tc和Cr的顺序取决于模拟物的化学性质,优选的还原途径和固相产物,例如磁铁矿(Fe 3 O 4)与针铁矿(α-FeOOH)。低pH和低Cr浓度有利于Tc的快速去除,XRD和XAS证实了Fe 3 O 4的存在。容易将还原的Tc(IV)纳入其结构。高pH,高Cr浓度和其他共混成分的存在有利于在反应早期(<1 h)异质去除Tc,且去除速率通常快于对Cr的去除速率。在> 1 h的反应时间,由于Cr的均匀去除开始占主导地位,因此Tc的去除速度变慢,同时固相反应产物FeOOH的形成增加。这些结果表明,对于复杂的高pH废物流,Fe(OH)2(s)处理后第一个小时内的Tc去除对于完全降低Tc和改善矿物质固定性是必要的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号