当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selenate Sorption by Hydrated Calcium Aluminate (AFm): Evidence for Sorption Reversibility and Implication for the Modeling of Anion Retention
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acsearthspacechem.9b00286 Sylvain Grangeon 1 , Nicolas C.M. Marty 1 , Nicolas Maubec 1 , Fabienne Warmont 2 , Francis Claret 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acsearthspacechem.9b00286 Sylvain Grangeon 1 , Nicolas C.M. Marty 1 , Nicolas Maubec 1 , Fabienne Warmont 2 , Francis Claret 1
Affiliation
|
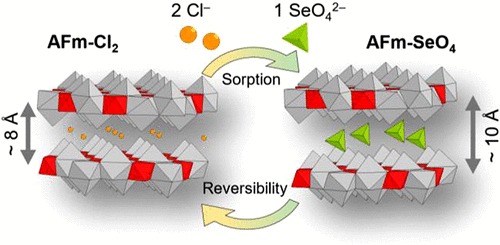
The mechanisms of selenate (SeO42–) sorption by Friedel’s salt (AFm-Cl2), a layered double hydroxide phase found in (altered) cement-based materials, were studied using a combination of wet chemistry, electron probe, X-ray scattering, and transmission electron microscopy techniques. Flowing a solution containing SeO42– through a suspension containing AFm-Cl2 in a dedicated chemical reactor led to several chemical and crystallographic modifications that were quantified by geochemical modeling of the wet chemistry data and analysis of the X-ray diffraction and scattering data in the reciprocal and real spaces. It was found that SeO42– replaced Cl– in the interlayer at the same crystallographic site to form monoselenate (AFm-SeO4) with an exchange stoichiometry that depended on the charges of the anions. This replacement was accompanied by an increase of the layer-to-layer distance. Sorption reversibility, a key parameter in being able to distinguish between adsorption and other uptake mechanisms, such as coprecipitation or structural incorporation, was studied by contacting the newly formed AFm-SeO4 with a Cl-rich solution. In this case, the Cl–/SeO42– exchange reaction was found to be reversible, following the same stoichiometry as the forward reaction with two Cl– replacing one SeO42– in the interlayer, and caused a reduction in the layer-to-layer distance. This study shows that AFm efficiently traps anions such as SeO42– through adsorption reaction and may thus play a significant role in retarding the migration of anionic contaminants through cement-based material and also that the adsorbed anions may be later released in case of a solution chemistry change.
中文翻译:

水合铝酸钙(AFm)的硒酸盐吸附:吸附可逆性的证据以及对阴离子保留的建模的意义
利用湿化学,电子探针,X-射线的组合研究了Friedel盐(AFm-Cl 2)吸附硒酸盐(SeO 4 2-)的机理,Friedel盐(AFm-Cl 2)是在(改变的)水泥基材料中发现的层状双氢氧化物相。射线散射和透射电子显微镜技术。在专用化学反应器中,将含有SeO 4 2–的溶液通过含有AFm-Cl 2的悬浮液,导致了几种化学和晶体学修饰,这些修饰通过湿化学数据的地球化学建模以及X射线衍射和散射数据的分析进行了量化。在互惠和真实的空间中。发现SeO 4 2 –取代了Cl –在中间结晶位置的夹层中形成单硒酸酯(AFm-SeO 4),其交换化学计量取决于阴离子的电荷。这种替换伴随着层到层距离的增加。通过使新形成的AFm-SeO 4与富含Cl的溶液接触,研究了吸附可逆性,这是能够区分吸附和其他吸收机制(例如共沉淀或结构结合)的关键参数。在此情况下,氯- /的SeO 4 2-交换反应被认为是可逆的,按照相同的化学计量与两个氯正向反应-替换一个搜索引擎优化4 2-在中间层中,并且导致层到层距离的减小。这项研究表明,AFm通过吸附反应有效地捕获了诸如SeO 4 2–的阴离子,因此可能在抑制阴离子污染物通过水泥基材料的迁移方面发挥了重要作用,并且在吸附离子的情况下,吸附的阴离子可能稍后释放。溶液化学变化。
更新日期:2020-01-10
中文翻译:

水合铝酸钙(AFm)的硒酸盐吸附:吸附可逆性的证据以及对阴离子保留的建模的意义
利用湿化学,电子探针,X-射线的组合研究了Friedel盐(AFm-Cl 2)吸附硒酸盐(SeO 4 2-)的机理,Friedel盐(AFm-Cl 2)是在(改变的)水泥基材料中发现的层状双氢氧化物相。射线散射和透射电子显微镜技术。在专用化学反应器中,将含有SeO 4 2–的溶液通过含有AFm-Cl 2的悬浮液,导致了几种化学和晶体学修饰,这些修饰通过湿化学数据的地球化学建模以及X射线衍射和散射数据的分析进行了量化。在互惠和真实的空间中。发现SeO 4 2 –取代了Cl –在中间结晶位置的夹层中形成单硒酸酯(AFm-SeO 4),其交换化学计量取决于阴离子的电荷。这种替换伴随着层到层距离的增加。通过使新形成的AFm-SeO 4与富含Cl的溶液接触,研究了吸附可逆性,这是能够区分吸附和其他吸收机制(例如共沉淀或结构结合)的关键参数。在此情况下,氯- /的SeO 4 2-交换反应被认为是可逆的,按照相同的化学计量与两个氯正向反应-替换一个搜索引擎优化4 2-在中间层中,并且导致层到层距离的减小。这项研究表明,AFm通过吸附反应有效地捕获了诸如SeO 4 2–的阴离子,因此可能在抑制阴离子污染物通过水泥基材料的迁移方面发挥了重要作用,并且在吸附离子的情况下,吸附的阴离子可能稍后释放。溶液化学变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号