当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Complicated Relationship: Glycosylation, Ca(II), and Primary Sequence Affect the Interactions and Kinetics between Two Model Mollusk Shell Intracrystalline Nacre Proteins.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.biochem.9b00867 Jose Juan-Colas 1 , Yong Seob Jung 2 , Steven Johnson 1 , John Spencer Evans 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.biochem.9b00867 Jose Juan-Colas 1 , Yong Seob Jung 2 , Steven Johnson 1 , John Spencer Evans 2
Affiliation
|
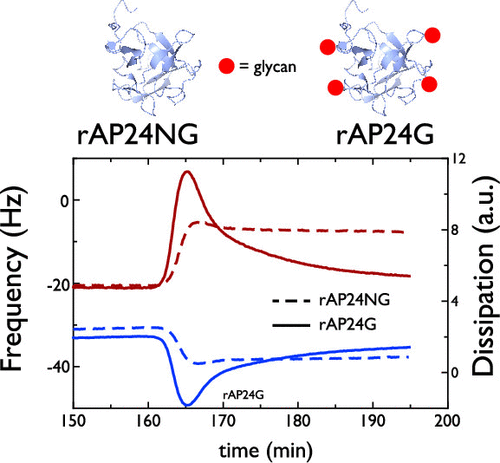
The formation of the mollusk shell requires the participation of proteins, many of which may be interactive with one another. We examined a model protein pair system from the mollusk Haliotis rufescens, wherein we probed the interactions between recombinant forms of two major nacre layer proteins, AP7, and the glycoprotein, AP24. Here, the focus was on the impact that the AP24 glycosylation and primary sequence had on AP24-AP7 binding. We find that both the glycosylated and nonglycosylated variants of AP24 bound to AP7 but with different quantities, kinetics, and internal rearrangements. Moreover, the binding of AP7 with nonglycosylated and glycosylated AP24 was found to be Ca(II)-dependent and -independent, respectively. Yet both variants of AP24 combine with AP7 to form hybrid hydrogel particles that are similar in their physical properties. Thus, AP7 and AP24 protein sequences are interactive and form hydrogels, but the interactions are tuned by glycosylation and Ca(II). These features may have an impact on the nacre matrix formation.
中文翻译:

复杂的关系:糖基化,Ca(II)和主要序列影响两个模型软体动物壳内结晶珍珠母蛋白之间的相互作用和动力学。
软体动物壳的形成需要蛋白质的参与,其中许多蛋白质可能彼此相互作用。我们检查了来自软体动物Haliotis rufescens的模型蛋白质对系统,其中我们研究了两种主要珍珠层蛋白AP7和糖蛋白AP24的重组形式之间的相互作用。在这里,重点是AP24糖基化和一级序列对AP24-AP7结合的影响。我们发现AP24的糖基化和非糖基化的变体都绑定到AP7,但具有不同的数量,动力学和内部重排。此外,发现AP7与非糖基化的和糖基化的AP24的结合分别是Ca(II)依赖性和非依赖性的。然而,AP24的两个变体都与AP7结合在一起,形成了物理性质相似的杂化水凝胶颗粒。因此,AP7和AP24蛋白质序列是相互作用的并形成水凝胶,但相互作用通过糖基化和Ca(II)进行调节。这些特征可能对珍珠母基质的形成有影响。
更新日期:2020-01-04
中文翻译:

复杂的关系:糖基化,Ca(II)和主要序列影响两个模型软体动物壳内结晶珍珠母蛋白之间的相互作用和动力学。
软体动物壳的形成需要蛋白质的参与,其中许多蛋白质可能彼此相互作用。我们检查了来自软体动物Haliotis rufescens的模型蛋白质对系统,其中我们研究了两种主要珍珠层蛋白AP7和糖蛋白AP24的重组形式之间的相互作用。在这里,重点是AP24糖基化和一级序列对AP24-AP7结合的影响。我们发现AP24的糖基化和非糖基化的变体都绑定到AP7,但具有不同的数量,动力学和内部重排。此外,发现AP7与非糖基化的和糖基化的AP24的结合分别是Ca(II)依赖性和非依赖性的。然而,AP24的两个变体都与AP7结合在一起,形成了物理性质相似的杂化水凝胶颗粒。因此,AP7和AP24蛋白质序列是相互作用的并形成水凝胶,但相互作用通过糖基化和Ca(II)进行调节。这些特征可能对珍珠母基质的形成有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号