当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Simulations of the Hypoxia-Inducible Factor PAS-B Domain Confirm That Internally Bound Water Molecules Function To Stabilize the Protein Core for Ligand Binding.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.biochem.9b00872 Andrew S Chong 1 , Peter C Anderson 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.biochem.9b00872 Andrew S Chong 1 , Peter C Anderson 1
Affiliation
|
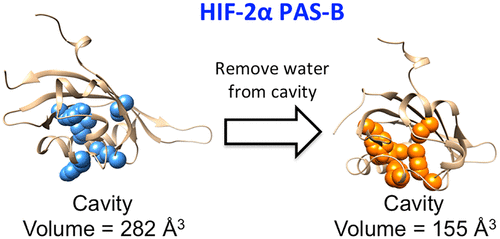
Hypoxia-inducible factors (HIFs) are heterodimeric transcription factors that are induced in many cancer types, promoting tumor-forming pathways. The HIF-2α PAS-B domain of the human HIF-2 complex contains a large buried hydrophilic cavity that is filled with eight ordered water molecules, making the domain a promising drug target. Recent work has led to the hypothesis that these buried water molecules act as an internal scaffold that stabilizes the buried cavity, holding it open to ease ligand binding. Here we show that extensive molecular dynamics simulations of the HIF-2α PAS-B domain strongly support this hypothesis. If water is prevented from entering the buried cavity, several amino acid side chains lining the cavity undergo a major conformational change that shrinks the cavity volume by an average of ∼45% relative to the hydrated cavity. The structural change within the cavity is due mainly to side chain rearrangements; the absence of bound water has only minor effects on the protein backbone conformation. Moreover, when the cavity lacks internally bound water, the side chain of Tyr307 rotates such that it blocks a major route for entry of the ligand into the cavity, greatly raising the free energy barrier for binding of an artificial ligand compared to when the cavity contains water. Finally, there is no difference in global protein stability of PAS-B when the cavity lacks water, as indicated by the same degree of protein flexibility and identical predicted melting temperatures. These findings help to explain further how water stabilizes large internal cavities, a relatively underexamined feature of proteins.
中文翻译:

缺氧诱导因子PAS-B结构域的分子动力学模拟证实,内部结合的水分子具有稳定配体结合蛋白核心的功能。
缺氧诱导因子(HIFs)是异源二聚体转录因子,可在多种癌症类型中诱导,从而促进肿瘤形成途径。人类HIF-2复合物的HIF-2αPAS-B结构域包含一个大的隐性亲水腔,该腔中充满了8个有序的水分子,使该结构域成为有希望的药物靶标。最近的工作提出了这样的假设,即这些埋藏的水分子充当内部支架,可以稳定埋藏的空腔,使其保持开放状态以促进配体结合。在这里,我们证明了HIF-2αPAS-B域的广泛分子动力学模拟强烈支持了这一假设。如果阻止水进入掩埋腔,则衬在腔内的几个氨基酸侧链会发生主要构象变化,相对于水合腔,腔的体积平均缩小约45%。腔内的结构变化主要是由于侧链重排所致。结合水的缺乏对蛋白质主链构象只有很小的影响。此外,当空腔中缺乏内部结合的水时,Tyr307的侧链旋转,从而阻止了配体进入空腔的主要途径,与空腔中包含的相比,大大提高了结合人工配体的自由能垒水。最后,当空腔缺少水时,PAS-B的整体蛋白质稳定性没有差异,这由相同程度的蛋白质柔韧性和相同的预测熔化温度所表明。这些发现有助于进一步解释水如何稳定大内腔,而大内腔是蛋白质相对不足的特征。
更新日期:2020-01-07
中文翻译:

缺氧诱导因子PAS-B结构域的分子动力学模拟证实,内部结合的水分子具有稳定配体结合蛋白核心的功能。
缺氧诱导因子(HIFs)是异源二聚体转录因子,可在多种癌症类型中诱导,从而促进肿瘤形成途径。人类HIF-2复合物的HIF-2αPAS-B结构域包含一个大的隐性亲水腔,该腔中充满了8个有序的水分子,使该结构域成为有希望的药物靶标。最近的工作提出了这样的假设,即这些埋藏的水分子充当内部支架,可以稳定埋藏的空腔,使其保持开放状态以促进配体结合。在这里,我们证明了HIF-2αPAS-B域的广泛分子动力学模拟强烈支持了这一假设。如果阻止水进入掩埋腔,则衬在腔内的几个氨基酸侧链会发生主要构象变化,相对于水合腔,腔的体积平均缩小约45%。腔内的结构变化主要是由于侧链重排所致。结合水的缺乏对蛋白质主链构象只有很小的影响。此外,当空腔中缺乏内部结合的水时,Tyr307的侧链旋转,从而阻止了配体进入空腔的主要途径,与空腔中包含的相比,大大提高了结合人工配体的自由能垒水。最后,当空腔缺少水时,PAS-B的整体蛋白质稳定性没有差异,这由相同程度的蛋白质柔韧性和相同的预测熔化温度所表明。这些发现有助于进一步解释水如何稳定大内腔,而大内腔是蛋白质相对不足的特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号