当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insights into the Binding of Natural Pyrimidine-Based Inhibitors of Class II Aminoacyl-tRNA Synthetases.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acschembio.9b00887 Luping Pang 1, 2 , Manesh Nautiyal 2 , Steff De Graef 1 , Bharat Gadakh 2 , Valentina Zorzini 3 , Anastassios Economou 3 , Sergei V Strelkov 1 , Arthur Van Aerschot 2 , Stephen D Weeks 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acschembio.9b00887 Luping Pang 1, 2 , Manesh Nautiyal 2 , Steff De Graef 1 , Bharat Gadakh 2 , Valentina Zorzini 3 , Anastassios Economou 3 , Sergei V Strelkov 1 , Arthur Van Aerschot 2 , Stephen D Weeks 1
Affiliation
|
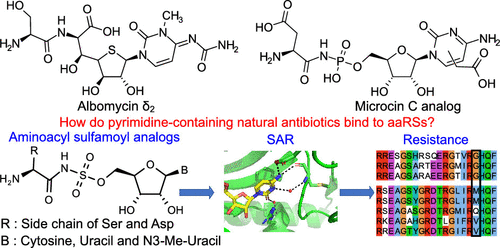
The pyrimidine-containing Trojan horse antibiotics albomycin and a recently discovered cytidine-containing microcin C analog target the class II seryl- and aspartyl-tRNA synthetases (serRS and aspRS), respectively. The active components of these compounds are competitive inhibitors that mimic the aminoacyl-adenylate intermediate. How they effectively substitute for the interactions mediated by the canonical purine group is unknown. Employing nonhydrolyzable aminoacyl-sulfamoyl nucleosides substituting the base with cytosine, uracil, and N3-methyluracil the structure-activity relationship of the natural compounds was evaluated. In vitro using E. coli serRS and aspRS, the best compounds demonstrated IC50 values in the low nanomolar range, with a clear preference for cytosine or N3-methyluracil over uracil. X-ray crystallographic structures of K. pneumoniae serRS and T. thermophilus aspRS in complex with the compounds showed the contribution of structured waters and residues in the conserved motif-2 loop in defining base preference. Utilizing the N3-methyluracil bound serRS structure, MD simulations of the fully modified albomycin base were performed to identify the interacting network that drives stable association. This analysis pointed to key interactions with a methionine in the motif-2 loop. Interestingly, this residue is mutated to a glycine in a second serRS (serRS2) found in albomycin-producing actinobacteria possessing self-immunity to this antibiotic. A comparative study demonstrated that serRS2 is poorly inhibited by the pyrimidine-containing intermediate analogs, and an equivalent mutation in E. coli serRS significantly decreased the affinity of the cytosine congener. These findings highlight the crucial role of dynamics and solvation of the motif-2 loop in modulating the binding of the natural antibiotics.
中文翻译:

结构的洞察力,基于II类氨酰基-tRNA合成酶的天然基于嘧啶的抑制剂的结合。
含嘧啶的特洛伊木马抗生素阿尔布霉素和最近发现的含胞苷的微素C类似物分别靶向II类丝氨酸和天冬氨酰tRNA合成酶(serRS和aspRS)。这些化合物的活性成分是竞争性抑制剂,可模仿氨基酰基-腺苷酸中间体。他们如何有效替代经典嘌呤基团介导的相互作用尚不清楚。使用不可水解的氨基酰基氨磺酰酰基核苷用胞嘧啶,尿嘧啶和N3-甲基尿嘧啶替代碱基,评估了天然化合物的构效关系。在体外使用大肠杆菌serRS和aspRS,最好的化合物显示出低纳摩尔范围内的IC50值,与尿嘧啶相比,胞嘧啶或N3-甲基尿嘧啶明显偏重。K的X射线晶体结构 肺炎病毒serRS和嗜热链球菌aspRS与化合物的复合物显示结构化水和保守基序2环中的残基在定义碱基偏爱方面的贡献。利用N3-甲基尿嘧啶结合的serRS结构,对完全修饰的阿波霉素碱基进行MD模拟,以鉴定驱动稳定缔合的相互作用网络。该分析指出了在motif-2环中与蛋氨酸的关键相互作用。有趣的是,该残基在产生对这种抗生素具有自身免疫性的产生造霉素的放线菌中发现的第二个serRS(serRS2)中突变为甘氨酸。一项比较研究表明,serRS2很难被含嘧啶的中间体类似物抑制,并且大肠杆菌serRS中的等效突变会显着降低胞嘧啶同源物的亲和力。
更新日期:2020-01-09
中文翻译:

结构的洞察力,基于II类氨酰基-tRNA合成酶的天然基于嘧啶的抑制剂的结合。
含嘧啶的特洛伊木马抗生素阿尔布霉素和最近发现的含胞苷的微素C类似物分别靶向II类丝氨酸和天冬氨酰tRNA合成酶(serRS和aspRS)。这些化合物的活性成分是竞争性抑制剂,可模仿氨基酰基-腺苷酸中间体。他们如何有效替代经典嘌呤基团介导的相互作用尚不清楚。使用不可水解的氨基酰基氨磺酰酰基核苷用胞嘧啶,尿嘧啶和N3-甲基尿嘧啶替代碱基,评估了天然化合物的构效关系。在体外使用大肠杆菌serRS和aspRS,最好的化合物显示出低纳摩尔范围内的IC50值,与尿嘧啶相比,胞嘧啶或N3-甲基尿嘧啶明显偏重。K的X射线晶体结构 肺炎病毒serRS和嗜热链球菌aspRS与化合物的复合物显示结构化水和保守基序2环中的残基在定义碱基偏爱方面的贡献。利用N3-甲基尿嘧啶结合的serRS结构,对完全修饰的阿波霉素碱基进行MD模拟,以鉴定驱动稳定缔合的相互作用网络。该分析指出了在motif-2环中与蛋氨酸的关键相互作用。有趣的是,该残基在产生对这种抗生素具有自身免疫性的产生造霉素的放线菌中发现的第二个serRS(serRS2)中突变为甘氨酸。一项比较研究表明,serRS2很难被含嘧啶的中间体类似物抑制,并且大肠杆菌serRS中的等效突变会显着降低胞嘧啶同源物的亲和力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号