当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of 4,5,6,7‐tetrahydrothieno[2,3‐c]pyridine–based β‐aminonitriles and their derivatives: β‐amino carboxamides, (thio)ureas, and tetracycles
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-23 , DOI: 10.1002/jhet.3800 Ramóna Madácsi 1, 2 , Péter Traj 2 , László Hackler 1 , Lajos I. Nagy 1 , Beáta Kari 1 , László G. Puskás 1 , Iván Kanizsai 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-23 , DOI: 10.1002/jhet.3800 Ramóna Madácsi 1, 2 , Péter Traj 2 , László Hackler 1 , Lajos I. Nagy 1 , Beáta Kari 1 , László G. Puskás 1 , Iván Kanizsai 1
Affiliation
|
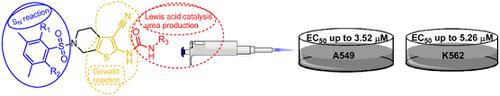
The preparation and cytotoxic characterization of 4,5,6,7‐tetrahydrothieno[2,3‐c]pyridine–based β‐aminonitriles, β‐amino carboxamides, and their (thio)urea and annulated derivatives were accomplished. Following a synthetic route involving Gewald three‐component reactions (G‐3CR) and a Lewis acid–catalyzed iso (thio)cyanate coupling, 30 compounds were prepared for antitumor evaluation. For derivatizations, a catalytic amount of CuOAc2 (20 mol%) was essential for improving the reactivity of either the C‐2 amino function of thiophene or isocyanates. The synthesized analogues demonstrated a weak to moderate antitumor activity in a low micromolar range against A549 and K562 cancer cell lines.
中文翻译:

4,5,6,7-四氢噻吩并[2,3-c]吡啶基β-氨基腈及其衍生物:β-氨基羧酰胺,(硫)脲和四环化合物的合成和生物学评估
完成了4,5,6,7-四氢噻吩并[2,3- c ]吡啶基的β-氨基腈,β-氨基羧酰胺及其(硫代)尿素和环状衍生物的制备和细胞毒性表征。按照涉及Gewald三组分反应(G-3CR)和路易斯酸催化的异(硫)氰酸酯偶联的合成路线,制备了30种化合物用于抗肿瘤评估。对于衍生化,催化量的CuOAc 2(20 mol%)对于提高噻吩或异氰酸酯的C-2氨基官能团的反应性至关重要。合成的类似物在低微摩尔范围内显示出对A549和K562癌细胞系的弱至中度抗肿瘤活性。
更新日期:2019-12-23
中文翻译:

4,5,6,7-四氢噻吩并[2,3-c]吡啶基β-氨基腈及其衍生物:β-氨基羧酰胺,(硫)脲和四环化合物的合成和生物学评估
完成了4,5,6,7-四氢噻吩并[2,3- c ]吡啶基的β-氨基腈,β-氨基羧酰胺及其(硫代)尿素和环状衍生物的制备和细胞毒性表征。按照涉及Gewald三组分反应(G-3CR)和路易斯酸催化的异(硫)氰酸酯偶联的合成路线,制备了30种化合物用于抗肿瘤评估。对于衍生化,催化量的CuOAc 2(20 mol%)对于提高噻吩或异氰酸酯的C-2氨基官能团的反应性至关重要。合成的类似物在低微摩尔范围内显示出对A549和K562癌细胞系的弱至中度抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号