当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable Mesoionic N-Heterocyclic Olefins (mNHOs).
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-21 , DOI: 10.1002/anie.201914571 Max M Hansmann 1, 2 , Patrick W Antoni 2 , Henner Pesch 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-21 , DOI: 10.1002/anie.201914571 Max M Hansmann 1, 2 , Patrick W Antoni 2 , Henner Pesch 2
Affiliation
|
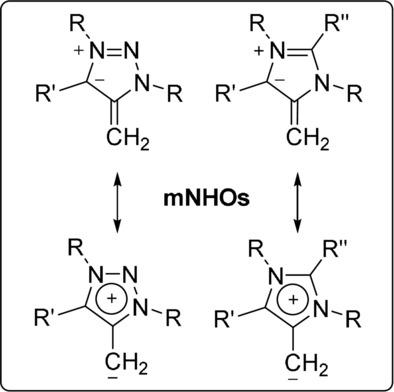
We report a new class of stable mesoionic N-heterocyclic olefins, featuring a highly polarized (strongly ylidic) double bond. The ground-state structure cannot be described through an uncharged mesomeric Lewis-structure, thereby structurally distinguishing them from traditional N-heterocyclic olefins (NHOs). mNHOs can easily be obtained through deprotonation of the corresponding methylated N,N'-diaryl-1,2,3-triazolium and N,N'-diaryl-imidazolium salts, respectively. In their reactivity, they represent strong σ-donor ligands as shown by their coordination complexes of rhodium and boron. Their calculated proton affinities, their experimentally derived basicities (competition experiments), as well as donor abilities (Tolman electronic parameter; TEP) exceed the so far reported class of NHOs.
中文翻译:

稳定的介离子 N-杂环烯烃 (mNHO)。
我们报告了一类新的稳定介离子N-杂环烯烃,具有高度极化(强叶立)双键。基态结构不能通过不带电荷的介观路易斯结构来描述,因此在结构上将它们与传统的N-杂环烯烃(NHO)区分开来。mNHOs 可以通过相应的甲基化 N,N'-二芳基-1,2,3-三唑鎓盐和 N,N'-二芳基-咪唑鎓盐分别去质子化来轻松获得。在它们的反应性中,它们代表强σ-供体配体,如铑和硼的配位络合物所示。它们计算出的质子亲和力、实验得出的碱度(竞争实验)以及供体能力(托尔曼电子参数;TEP)超过了迄今为止报道的 NHO 类别。
更新日期:2020-01-27
中文翻译:

稳定的介离子 N-杂环烯烃 (mNHO)。
我们报告了一类新的稳定介离子N-杂环烯烃,具有高度极化(强叶立)双键。基态结构不能通过不带电荷的介观路易斯结构来描述,因此在结构上将它们与传统的N-杂环烯烃(NHO)区分开来。mNHOs 可以通过相应的甲基化 N,N'-二芳基-1,2,3-三唑鎓盐和 N,N'-二芳基-咪唑鎓盐分别去质子化来轻松获得。在它们的反应性中,它们代表强σ-供体配体,如铑和硼的配位络合物所示。它们计算出的质子亲和力、实验得出的碱度(竞争实验)以及供体能力(托尔曼电子参数;TEP)超过了迄今为止报道的 NHO 类别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号