当前位置:
X-MOL 学术
›
Nat. Protoc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential phosphoproteomics and N-glycoproteomics of plasma-derived extracellular vesicles.
Nature Protocols ( IF 13.1 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41596-019-0260-5 Hillary Andaluz Aguilar 1 , Anton B Iliuk 2, 3 , I-Hsuan Chen 3 , W Andy Tao 1, 2, 3, 4
Nature Protocols ( IF 13.1 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41596-019-0260-5 Hillary Andaluz Aguilar 1 , Anton B Iliuk 2, 3 , I-Hsuan Chen 3 , W Andy Tao 1, 2, 3, 4
Affiliation
|
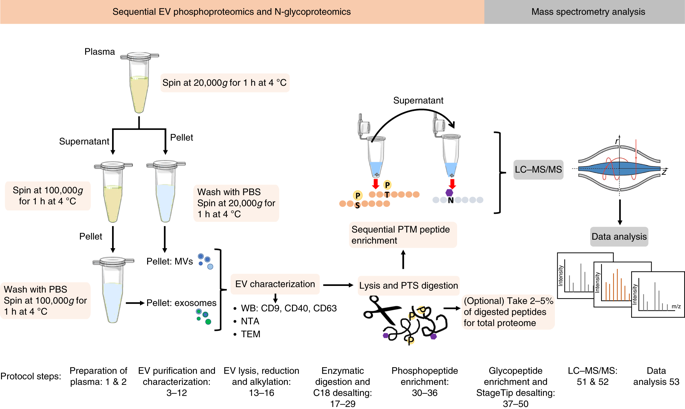
Extracellular vesicles (EVs) are increasingly being recognized as important vehicles for intercellular communication and as promising sources for biomarker discovery. Because the state of protein post-translational modifications (PTMs) such as phosphorylation and glycosylation can be a key determinant of cellular physiology, comprehensive characterization of protein PTMs in EVs can be particularly valuable for early-stage diagnostics and monitoring of disease status. However, the analysis of PTMs in EVs has been complicated by limited amounts of purified EVs, low-abundance PTM proteins, and interference from proteins and metabolites in biofluids. Recently, we developed an approach to isolate phosphoproteins and glycoproteins in EVs from small volumes of human plasma that enabled us to identify nearly 10,000 unique phosphopeptides and 1,500 unique N-glycopeptides. The approach demonstrated the feasibility of using these data to identify potential markers to differentiate disease from healthy states. Here we present an updated workflow to sequentially isolate phosphopeptides and N-glycopeptides, enabling multiple PTM analyses of the same clinical samples. In this updated workflow, we have improved the reproducibility and efficiency of EV isolation, protein extraction, and phosphopeptide/N-glycopeptide enrichment to achieve sensitive analyses of low-abundance PTMs in EVs isolated from 1 mL of plasma. The modularity of the workflow also allows for the characterization of phospho- or glycopeptides only and enables additional analysis of total proteomes and other PTMs of interest. After blood collection, the protocol takes 2 d, including EV isolation, PTM/peptide enrichment, mass spectrometry analysis, and data quantification.
中文翻译:

血浆来源的细胞外囊泡的序列磷酸蛋白质组学和 N-糖蛋白质组学。
细胞外囊泡 (EV) 越来越被认为是细胞间通讯的重要载体和生物标志物发现的有希望的来源。由于蛋白质翻译后修饰 (PTM) 的状态(如磷酸化和糖基化)可能是细胞生理学的关键决定因素,因此对 EV 中蛋白质 PTM 的综合表征对于早期诊断和监测疾病状态特别有价值。然而,由于纯化的 EV 数量有限、PTM 蛋白丰度低以及生物流体中蛋白质和代谢物的干扰,EV 中 PTM 的分析变得复杂。最近,我们开发了一种从少量人血浆中分离 EV 中的磷酸蛋白和糖蛋白的方法,这使我们能够识别近 10,000 种独特的磷酸肽和 1,500 种独特的 N-糖肽。该方法证明了使用这些数据来识别潜在标志物以区分疾病和健康状态的可行性。在这里,我们提出了一个更新的工作流程,以顺序分离磷酸肽和 N-糖肽,从而实现对相同临床样本的多个 PTM 分析。在这个更新的工作流程中,我们提高了 EV 分离、蛋白质提取和磷酸肽/N-糖肽富集的可重复性和效率,以实现对从 1 mL 血浆中分离的 EV 中低丰度 PTM 的灵敏分析。工作流程的模块化还允许仅表征磷酸肽或糖肽,并能够对总蛋白质组和其他感兴趣的 PTM 进行额外分析。采血后,该方案需要 2 天,包括 EV 分离、PTM/肽富集、
更新日期:2019-12-21
中文翻译:

血浆来源的细胞外囊泡的序列磷酸蛋白质组学和 N-糖蛋白质组学。
细胞外囊泡 (EV) 越来越被认为是细胞间通讯的重要载体和生物标志物发现的有希望的来源。由于蛋白质翻译后修饰 (PTM) 的状态(如磷酸化和糖基化)可能是细胞生理学的关键决定因素,因此对 EV 中蛋白质 PTM 的综合表征对于早期诊断和监测疾病状态特别有价值。然而,由于纯化的 EV 数量有限、PTM 蛋白丰度低以及生物流体中蛋白质和代谢物的干扰,EV 中 PTM 的分析变得复杂。最近,我们开发了一种从少量人血浆中分离 EV 中的磷酸蛋白和糖蛋白的方法,这使我们能够识别近 10,000 种独特的磷酸肽和 1,500 种独特的 N-糖肽。该方法证明了使用这些数据来识别潜在标志物以区分疾病和健康状态的可行性。在这里,我们提出了一个更新的工作流程,以顺序分离磷酸肽和 N-糖肽,从而实现对相同临床样本的多个 PTM 分析。在这个更新的工作流程中,我们提高了 EV 分离、蛋白质提取和磷酸肽/N-糖肽富集的可重复性和效率,以实现对从 1 mL 血浆中分离的 EV 中低丰度 PTM 的灵敏分析。工作流程的模块化还允许仅表征磷酸肽或糖肽,并能够对总蛋白质组和其他感兴趣的 PTM 进行额外分析。采血后,该方案需要 2 天,包括 EV 分离、PTM/肽富集、











































 京公网安备 11010802027423号
京公网安备 11010802027423号