当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Restricted and non-essential redundancy of RNAi and piRNA pathways in mouse oocytes.
PLOS Genetics ( IF 4.0 ) Pub Date : 2019-12-20 , DOI: 10.1371/journal.pgen.1008261 Eliska Taborska 1 , Josef Pasulka 1 , Radek Malik 1 , Filip Horvat 1, 2 , Irena Jenickova 3 , Zoe Jelić Matošević 2 , Petr Svoboda 1
PLOS Genetics ( IF 4.0 ) Pub Date : 2019-12-20 , DOI: 10.1371/journal.pgen.1008261 Eliska Taborska 1 , Josef Pasulka 1 , Radek Malik 1 , Filip Horvat 1, 2 , Irena Jenickova 3 , Zoe Jelić Matošević 2 , Petr Svoboda 1
Affiliation
|
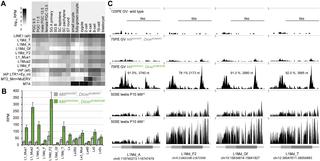
Germline genome defense evolves to recognize and suppress retrotransposons. One of defensive mechanisms is the PIWI-associated RNA (piRNA) pathway, which employs small RNAs for sequence-specific repression. The loss of the piRNA pathway in mice causes male sterility while females remain fertile. Unlike spermatogenic cells, mouse oocytes posses also RNA interference (RNAi), another small RNA pathway capable of retrotransposon suppression. To examine whether RNAi compensates the loss of the piRNA pathway, we produced a new RNAi pathway mutant DicerSOM and crossed it with a catalytically-dead mutant of Mili, an essential piRNA gene. Normal follicular and oocyte development in double mutants showed that RNAi does not suppress a strong ovarian piRNA knock-out phenotype. However, we observed redundant and non-redundant targeting of specific retrotransposon families illustrating stochasticity of recognition and targeting of invading retrotransposons. Intracisternal A Particle retrotransposon was mainly targeted by the piRNA pathway, MaLR and RLTR10 retrotransposons were targeted mainly by RNAi. Double mutants showed accumulations of LINE-1 retrotransposon transcripts. However, we did not find strong evidence for transcriptional activation and mobilization of retrotransposition competent LINE-1 elements suggesting that while both defense pathways are simultaneously expendable for ovarian oocyte development, yet another transcriptional silencing mechanism prevents mobilization of LINE-1 elements.
中文翻译:

小鼠卵母细胞中RNAi和piRNA途径的限制性和非必需冗余。
生殖系基因组防御进化为识别和抑制逆转座子。防御机制之一是PIWI相关RNA(piRNA)途径,该途径采用小RNA进行序列特异性抑制。小鼠中piRNA途径的缺失会导致雄性不育,而雌性则保持可育性。与生精细胞不同,小鼠卵母细胞也具有RNA干扰(RNAi),RNA干扰是另一个能够逆转转座子抑制的小RNA途径。为了检查RNAi是否补偿piRNA途径的缺失,我们制备了一个新的RNAi途径突变体DicerSOM,并将其与必不可少的piRNA基因Mili的催化死亡突变体杂交。双重突变体中正常的卵泡和卵母细胞发育表明,RNAi不抑制强烈的卵巢piRNA敲除表型。然而,我们观察到特定逆转录转座子家族的冗余和非冗余靶向,说明了识别的随机性和入侵逆转录转座子的靶向。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1逆转录转座子转录本的积累。但是,我们没有找到强有力的证据证明转录激活和逆转座感受态LINE-1元素动员,这表明虽然两种防御途径同时可促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1反转录转座子转录本的积累。但是,我们没有找到强有力的证据来证明转录激活和逆转录主管LINE-1元素的动员,这表明尽管两种防御途径都可同时促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1逆转录转座子转录本的积累。但是,我们没有找到强有力的证据来证明转录激活和逆转录主管LINE-1元素的动员,这表明尽管两种防御途径都可同时促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。
更新日期:2019-12-21
中文翻译:

小鼠卵母细胞中RNAi和piRNA途径的限制性和非必需冗余。
生殖系基因组防御进化为识别和抑制逆转座子。防御机制之一是PIWI相关RNA(piRNA)途径,该途径采用小RNA进行序列特异性抑制。小鼠中piRNA途径的缺失会导致雄性不育,而雌性则保持可育性。与生精细胞不同,小鼠卵母细胞也具有RNA干扰(RNAi),RNA干扰是另一个能够逆转转座子抑制的小RNA途径。为了检查RNAi是否补偿piRNA途径的缺失,我们制备了一个新的RNAi途径突变体DicerSOM,并将其与必不可少的piRNA基因Mili的催化死亡突变体杂交。双重突变体中正常的卵泡和卵母细胞发育表明,RNAi不抑制强烈的卵巢piRNA敲除表型。然而,我们观察到特定逆转录转座子家族的冗余和非冗余靶向,说明了识别的随机性和入侵逆转录转座子的靶向。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1逆转录转座子转录本的积累。但是,我们没有找到强有力的证据证明转录激活和逆转座感受态LINE-1元素动员,这表明虽然两种防御途径同时可促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1反转录转座子转录本的积累。但是,我们没有找到强有力的证据来证明转录激活和逆转录主管LINE-1元素的动员,这表明尽管两种防御途径都可同时促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。颅内A粒子逆转座子主要通过piRNA途径靶向,MaLR和RLTR10逆转座子主要通过RNAi靶向。双突变体显示LINE-1逆转录转座子转录本的积累。但是,我们没有找到强有力的证据来证明转录激活和逆转录主管LINE-1元素的动员,这表明尽管两种防御途径都可同时促进卵巢卵母细胞发育,但另一种转录沉默机制却阻止了LINE-1元素的动员。











































 京公网安备 11010802027423号
京公网安备 11010802027423号