当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single to Multiple Site Behavior of Metallocenes through C–H Activation for Olefin Polymerization: A Mechanistic Insight from DFT
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acscatal.9b03741 Jugal Kumawat 1 , Virendra Kumar Gupta 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acscatal.9b03741 Jugal Kumawat 1 , Virendra Kumar Gupta 1
Affiliation
|
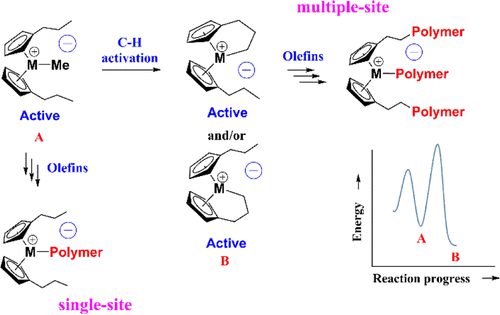
Density functional theory has been used to study single to multiple site behavior of metallocene catalysts for olefin polymerization using (CpPr)2Hf(R)2 and (CpPr)2Zr(R)2 (here, R = Me and n-butyl group) and boron activators B(C6F5)3 and [CPh3]+[B(C6F5)4]−. Detailed pathways were investigated for two steps: (i) catalyst activation and (ii) ethylene and 1-hexene polymerization. For the catalyst activation step, the C–H activation of Cp-substituents (here, the n-propyl group) has also been studied, which leads to another active site named “metal cyclic active site” in comparison to the conventional active site. Among these sites, the formation of a metal cyclic active site is slower with respect to the conventional active site. Additionally, two different routes have been explored for the catalyst activation, depending on the nature of activators, and observed that [CPh3]+[B(C6F5)4]− is a better activator with respect to B(C6F5)3. Moreover, complete insertion (primary and secondary) and termination (chain transfer to monomer and β-H elimination) steps have also been studied for both the active sites. These results suggest that the metal cyclic active site can also produce the ethylene and 1-hexene polymer, which defines the multiple site behavior of metallocene catalysts, which explains the broadening of molecular weight distribution of linear low-density polyethylene-produced metallocene catalysts. Along with hafnocene and zirconocene, the toluene-solvated hafnocene catalyst system has also been investigated for catalyst activation and ethylene polymerization for comparison study. These results indicate that the toluene-solvated hafnocene catalyst system is energetically more facile in comparison to other catalysts. Furthermore, the effect of solvents as well as dispersion interaction on catalyst activation steps has been studied for the (CpPr)2M(n-butyl)2 (M = Hf and Zr) catalyst systems and observed that the dispersion interaction played an important role in the catalyst activation process. In addition to this, the regio- and stereoselective behavior of 1-hexene monomer for the (CpPr)2Hf(Me)2 catalyst has been investigated, and it was found that the metal cyclic active site improves the stereoselective nature of the hafnocene catalyst. Therefore, the current chemical calculations provide insights into the nature of active sites in metallocene catalysts for olefin polymerization.
中文翻译:

通过C–H活化茂金属对烯烃的单点或多点行为:DFT的机理分析
密度泛函理论已被用于研究单个对金属茂催化剂的多个网站行为使用烯烃聚合(CP镨)2 HF(R)2和(CP镨)2锆(R)2(这里,R =我和ñ -丁基)和硼活化剂B(C 6 F 5)3和[CPh 3 ] + [B(C 6 F 5)4 ] -。研究了两个步骤的详细途径:(i)催化剂活化和(ii)乙烯和1-己烯聚合。在催化剂活化步骤中,还研究了Cp取代基的C–H活化(此处为正丙基),与传统的活性部位相比,这导致了另一个名为“金属环状活性部位”的活性部位。在这些位点中,金属环状活性位点的形成相对于常规活性位点较慢。此外,根据活化剂的性质,探索了两种不同的催化剂活化途径,并观察到[CPh 3 ] + [B(C 6 F 5)4 ] -相对于B(C 6 F 5)3是更好的活化剂。此外,还已经针对两个活性位点研究了完全插入(主要和次要)和终止(链转移至单体和β-H消除)步骤。这些结果表明金属环状活性位点也可以产生乙烯和1-己烯聚合物,这定义了茂金属催化剂的多位行为,这解释了线性低密度聚乙烯生产的茂金属催化剂的分子量分布的扩大。除ha和锆茂外,还研究了甲苯溶剂化的c茂催化剂体系用于催化剂活化和乙烯聚合的比较研究。这些结果表明,与其他催化剂相比,甲苯溶剂化的c茂催化剂体系在能量上更容易实现。此外,Pr)2 M(n-丁基)2(M = Hf和Zr)催化剂体系,并观察到分散相互作用在催化剂活化过程中起着重要作用。除此之外,还研究了1-己烯单体对(Cp Pr)2 Hf(Me)2催化剂的区域和立体选择性行为,发现金属环状活性位点改善了f的立体选择性。催化剂。因此,当前的化学计算提供了对于用于烯烃聚合的茂金属催化剂中活性位点的性质的见解。
更新日期:2020-01-10
中文翻译:

通过C–H活化茂金属对烯烃的单点或多点行为:DFT的机理分析
密度泛函理论已被用于研究单个对金属茂催化剂的多个网站行为使用烯烃聚合(CP镨)2 HF(R)2和(CP镨)2锆(R)2(这里,R =我和ñ -丁基)和硼活化剂B(C 6 F 5)3和[CPh 3 ] + [B(C 6 F 5)4 ] -。研究了两个步骤的详细途径:(i)催化剂活化和(ii)乙烯和1-己烯聚合。在催化剂活化步骤中,还研究了Cp取代基的C–H活化(此处为正丙基),与传统的活性部位相比,这导致了另一个名为“金属环状活性部位”的活性部位。在这些位点中,金属环状活性位点的形成相对于常规活性位点较慢。此外,根据活化剂的性质,探索了两种不同的催化剂活化途径,并观察到[CPh 3 ] + [B(C 6 F 5)4 ] -相对于B(C 6 F 5)3是更好的活化剂。此外,还已经针对两个活性位点研究了完全插入(主要和次要)和终止(链转移至单体和β-H消除)步骤。这些结果表明金属环状活性位点也可以产生乙烯和1-己烯聚合物,这定义了茂金属催化剂的多位行为,这解释了线性低密度聚乙烯生产的茂金属催化剂的分子量分布的扩大。除ha和锆茂外,还研究了甲苯溶剂化的c茂催化剂体系用于催化剂活化和乙烯聚合的比较研究。这些结果表明,与其他催化剂相比,甲苯溶剂化的c茂催化剂体系在能量上更容易实现。此外,Pr)2 M(n-丁基)2(M = Hf和Zr)催化剂体系,并观察到分散相互作用在催化剂活化过程中起着重要作用。除此之外,还研究了1-己烯单体对(Cp Pr)2 Hf(Me)2催化剂的区域和立体选择性行为,发现金属环状活性位点改善了f的立体选择性。催化剂。因此,当前的化学计算提供了对于用于烯烃聚合的茂金属催化剂中活性位点的性质的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号