当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 reactivity with Mg2NiH4 synthesized by in situ monitoring of mechanical milling.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05697a M L Grasso 1 , J Puszkiel 2 , F C Gennari 1 , A Santoru 3 , M Dornheim 3 , C Pistidda 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05697a M L Grasso 1 , J Puszkiel 2 , F C Gennari 1 , A Santoru 3 , M Dornheim 3 , C Pistidda 3
Affiliation
|
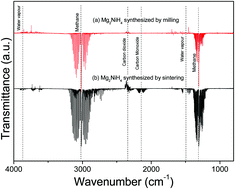
CO2 capture and conversion are a key research field for the transition towards an economy only based on renewable energy sources. In this regard, hydride materials are a potential option for CO2 methanation since they can provide hydrogen and act as a catalytic species. In this work, Mg2NiH4 complex hydride is synthesized by in situ monitoring of mechanical milling under a hydrogen atmosphere from a 2MgH2:Ni stoichiometric mixture. Temperature and pressure evolution is monitored, and the material is characterized, during milling in situ, thus providing a good insight into the synthesis process. The cubic polymorph of Mg2NiH4 (S.G. Fm3[combining macron]m) starts to be formed in the early beginning of the mechanical treatment due to the mechanical stress induced by the milling process. Then, after 25 hours of milling, Mg2NiH4 with a monoclinic (S.G. C12/c1) structure appears. The formation of the monoclinic polymorph is most likely related to the stress release that follows the continuous refinement of the material's microstructure. At the end of the milling process, after 60 hours, the as-milled material is composed of 90.8 wt% cubic Mg2NiH4, 5.7 wt% monoclinic Mg2NiH4, and 3.5 wt% remnant Ni. The as-milled Mg2NiH4 shows high reactivity for CO2 conversion into CH4. Under static conditions at 400 °C for 5 hours, the interactions between as-milled Mg2NiH4 and CO2 result in total CO2 consumption and in the formation of the catalytic system Ni-MgNi2-Mg2Ni/MgO. Experimental evidence and thermodynamic equilibrium calculations suggest that the global methanation mechanism takes place through the adsorption of C and the direct solid gasification towards CH4 formation.
中文翻译:

通过机械研磨的原位监测合成的Mg2NiH4的CO2反应性。
二氧化碳的捕获和转化是仅向可再生能源经济迈进的关键研究领域。在这方面,氢化物材料是CO2甲烷化的潜在选择,因为它们可以提供氢气并充当催化物质。在这项工作中,Mg2NiH4复合氢化物是通过在氢气气氛下从2MgH2:Ni化学计量混合物中对机械研磨进行原位监测而合成的。在原位研磨过程中,可以监控温度和压力的变化,并对材料进行表征,从而提供了对合成过程的深入了解。由于铣削过程引起的机械应力,Mg2NiH4的立方多晶型物(SG Fm3 [结合宏] m)开始在机械处理的早期开始形成。然后,在研磨25小时后,将Mg2NiH4与单斜晶(SG C12 / c1)结构出现。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程的最后,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机理是通过C的吸附和直接固体气化形成CH4发生的。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程结束时,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机制是通过C的吸附和直接固体气化形成CH4来实现的。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程的最后,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机理是通过C的吸附和直接固体气化形成CH4发生的。
更新日期:2020-01-09
中文翻译:

通过机械研磨的原位监测合成的Mg2NiH4的CO2反应性。
二氧化碳的捕获和转化是仅向可再生能源经济迈进的关键研究领域。在这方面,氢化物材料是CO2甲烷化的潜在选择,因为它们可以提供氢气并充当催化物质。在这项工作中,Mg2NiH4复合氢化物是通过在氢气气氛下从2MgH2:Ni化学计量混合物中对机械研磨进行原位监测而合成的。在原位研磨过程中,可以监控温度和压力的变化,并对材料进行表征,从而提供了对合成过程的深入了解。由于铣削过程引起的机械应力,Mg2NiH4的立方多晶型物(SG Fm3 [结合宏] m)开始在机械处理的早期开始形成。然后,在研磨25小时后,将Mg2NiH4与单斜晶(SG C12 / c1)结构出现。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程的最后,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机理是通过C的吸附和直接固体气化形成CH4发生的。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程结束时,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机制是通过C的吸附和直接固体气化形成CH4来实现的。单斜晶多晶型物的形成很可能与材料微观结构不断细化之后的应力释放有关。在研磨过程的最后,在60小时后,研磨后的材料由90.8 wt%的立方Mg2NiH4、5.7 wt%的单斜晶Mg2NiH4和3.5 wt%的残留Ni组成。研磨后的Mg2NiH4对CO2转化为CH4具有很高的反应性。在400°C的静态条件下5个小时,研磨后的Mg2NiH4与CO2之间的相互作用导致总的CO2消耗和催化体系Ni-MgNi2-Mg2Ni / MgO的形成。实验证据和热力学平衡计算表明,整体甲烷化机理是通过C的吸附和直接固体气化形成CH4发生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号