当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trisulfur radical anion-triggered stitching thienannulation: rapid access to largely π-extended thienoacenes
Chemical Science ( IF 7.6 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9sc05332h Feng Su 1 , Shuqi Chen 1 , Xiaogang Mo 1 , Kongchuan Wu 1 , Jiajun Wu 1 , Weidong Lin 1 , Zhiwei Lin 1 , Jianbin Lin 1, 2 , Hui-Jun Zhang 1 , Ting-Bin Wen 1
Chemical Science ( IF 7.6 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9sc05332h Feng Su 1 , Shuqi Chen 1 , Xiaogang Mo 1 , Kongchuan Wu 1 , Jiajun Wu 1 , Weidong Lin 1 , Zhiwei Lin 1 , Jianbin Lin 1, 2 , Hui-Jun Zhang 1 , Ting-Bin Wen 1
Affiliation
|
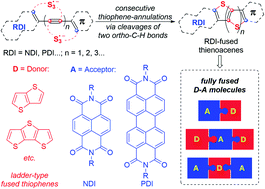
Largely π-extended rylene diimide-fused thienoacenes, a new family of fully fused electron donor–acceptor (D–A) molecules, have been readily synthesized by a novel trisulfur radical anion (S3˙−)-triggered stitching thienannulation strategy. The ladder-type fused thiophene cores are constructed in a stitching manner through multiple carbon–sulfur bond formation between acetylenic rylene dyes and S3˙−. A detailed mechanistic study of these stitching thienannulations unveiled the multiple reactivities of S3˙−. Physical properties of the newly formed D–A, A–D–A, and D–A–D type thienoacenes have also been investigated, which revealed their precisely controllable electronic properties.
中文翻译:

三硫自由基阴离子触发的缝合噻吩环化:快速获得大部分π延伸的噻吩并苯
大部分π延伸的萘嵌苯二酰亚胺稠合噻吩并苯是一种完全稠合的电子供体-受体(D-A)分子的新家族,已通过新型三硫自由基阴离子(S 3 ˙ -)触发的缝合噻吩环化策略轻松合成。梯型稠合噻吩核是通过炔属萘嵌苯染料和S 3 ˙ -之间形成多个碳硫键以缝合方式构建的。对这些缝合噻环作用的详细机制研究揭示了 S 3 ˙ −的多种反应性。新形成的D-A、A-D-A和D-A-D型噻吩并苯的物理性质也得到了研究,揭示了它们精确可控的电子性质。
更新日期:2020-02-13
中文翻译:

三硫自由基阴离子触发的缝合噻吩环化:快速获得大部分π延伸的噻吩并苯
大部分π延伸的萘嵌苯二酰亚胺稠合噻吩并苯是一种完全稠合的电子供体-受体(D-A)分子的新家族,已通过新型三硫自由基阴离子(S 3 ˙ -)触发的缝合噻吩环化策略轻松合成。梯型稠合噻吩核是通过炔属萘嵌苯染料和S 3 ˙ -之间形成多个碳硫键以缝合方式构建的。对这些缝合噻环作用的详细机制研究揭示了 S 3 ˙ −的多种反应性。新形成的D-A、A-D-A和D-A-D型噻吩并苯的物理性质也得到了研究,揭示了它们精确可控的电子性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号