当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azido-Desferrioxamine Siderophores as Functional Click-Chemistry Probes Generated in Culture upon Adding a Diazo-Transfer Reagent.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-20 , DOI: 10.1002/cbic.201900661 Michael P Gotsbacher 1 , Rachel Codd 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-20 , DOI: 10.1002/cbic.201900661 Michael P Gotsbacher 1 , Rachel Codd 1
Affiliation
|
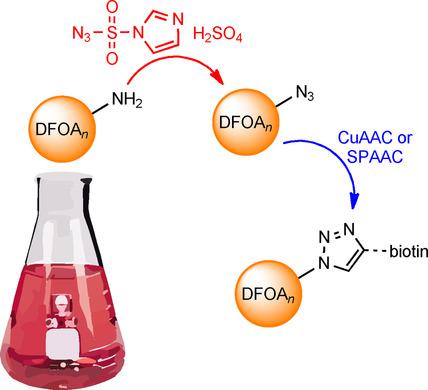
This work aimed to undertake the in situ conversion of the terminal amine groups of bacterial desferrioxamine (DFO) siderophores, including desferrioxamine B (DFOB), to azide groups to enable downstream click chemistry. Initial studies trialed a precursor-directed biosynthesis (PDB) approach. Supplementing Streptomyces pilosus culture with blunt-end azido/amine non-native substrates designed to replace 1,5-diaminopentane as the native diamine substrate in the terminal amine position of DFOB did not produce azido-DFOB. Addition of the diazo-transfer reagent imidazole-1-sulfonyl azide hydrogen sulfate to spent S. pilosus medium that had been cultured in the presence of 1,4-diaminobutane, as a viable native substrate to expand the suite of native DFO-type siderophores, successfully generated the cognate suite of azido-DFO analogues. CuI -mediated or strain-promoted CuI -free click chemistry reactions between this minimally processed mixture and the appropriate alkyne-bearing biotin reagents produced the cognate suite of 1,4-disubstituted triazole-linked DFO-biotin compounds as potential molecular probes, detected as FeIII -loaded species. The amine-to-azide transformation of amine-bearing natural products in complex mixtures by the direct addition of a diazo-transfer reagent to deliver functional click chemistry reagents adds to the toolbox for chemical proteomics, chemical biology, and drug discovery.
中文翻译:

添加重氮转移试剂后,在培养中产生的叠氮基-去铁胺胺铁载体作为功能性点击化学探针。
这项工作旨在进行细菌去铁氧胺(DFO)铁载体的端胺基团(包括去铁胺B(DFOB))的原位转化为叠氮基团的操作,以实现下游点击化学反应。最初的研究试用了前驱体定向生物合成(PDB)方法。用钝端叠氮基/胺非天然底物补充毛链霉菌培养物以取代1,5-二氨基戊烷作为DFOB末端胺位置的天然二胺底物不会产生叠氮基-DFOB。将重氮转移试剂咪唑-1-磺酰叠氮化硫酸氢盐添加到已在1,4-二氨基丁烷存在下培养的废毕赤酵母培养基中,作为可行的天然底物以扩展天然DFO型铁载体的套件,成功生成了相关的azido-DFO类似物套件。这种最少加工的混合物与适当的带有炔烃的生物素试剂之间的CuI介导的或菌株促进的CuI无点击化学反应产生了相关的1,4-二取代的三唑连接的DFO-生物素化合物作为潜在的分子探针,检测为FeIII负载的物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。被检测为FeIII负载物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。被检测为FeIII负载物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。
更新日期:2019-12-20
中文翻译:

添加重氮转移试剂后,在培养中产生的叠氮基-去铁胺胺铁载体作为功能性点击化学探针。
这项工作旨在进行细菌去铁氧胺(DFO)铁载体的端胺基团(包括去铁胺B(DFOB))的原位转化为叠氮基团的操作,以实现下游点击化学反应。最初的研究试用了前驱体定向生物合成(PDB)方法。用钝端叠氮基/胺非天然底物补充毛链霉菌培养物以取代1,5-二氨基戊烷作为DFOB末端胺位置的天然二胺底物不会产生叠氮基-DFOB。将重氮转移试剂咪唑-1-磺酰叠氮化硫酸氢盐添加到已在1,4-二氨基丁烷存在下培养的废毕赤酵母培养基中,作为可行的天然底物以扩展天然DFO型铁载体的套件,成功生成了相关的azido-DFO类似物套件。这种最少加工的混合物与适当的带有炔烃的生物素试剂之间的CuI介导的或菌株促进的CuI无点击化学反应产生了相关的1,4-二取代的三唑连接的DFO-生物素化合物作为潜在的分子探针,检测为FeIII负载的物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。被检测为FeIII负载物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。被检测为FeIII负载物种。通过直接添加重氮转移试剂以传递功能性点击化学试剂,将复杂混合物中的含胺天然产物进行胺-叠氮化物转化,从而为化学蛋白质组学,化学生物学和药物发现增加了工具箱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号