当前位置:
X-MOL 学术
›
Insect Biochem. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Weaponisation 'on the fly': Convergent recruitment of knottin and defensin peptide scaffolds into the venom of predatory assassin flies.
Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2019-12-21 , DOI: 10.1016/j.ibmb.2019.103310 Jiayi Jin 1 , Akello J Agwa 1 , Tibor G Szanto 2 , Agota Csóti 2 , Gyorgy Panyi 2 , Christina I Schroeder 1 , Andrew A Walker 1 , Glenn F King 1
Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2019-12-21 , DOI: 10.1016/j.ibmb.2019.103310 Jiayi Jin 1 , Akello J Agwa 1 , Tibor G Szanto 2 , Agota Csóti 2 , Gyorgy Panyi 2 , Christina I Schroeder 1 , Andrew A Walker 1 , Glenn F King 1
Affiliation
|
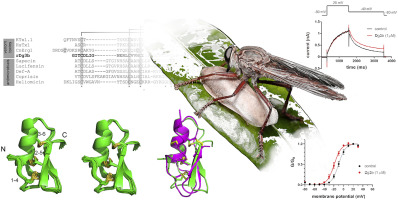
Many arthropod venom peptides have potential as bioinsecticides, drug leads, and pharmacological tools due to their specific neuromodulatory functions. Assassin flies (Asilidae) are a family of predaceous dipterans that produce a unique and complex peptide-rich venom for killing insect prey and deterring predators. However, very little is known about the structure and function of their venom peptides. We therefore used an E. coli periplasmic expression system to express four disulfide-rich peptides that we previously reported to exist in venom of the giant assassin fly Dolopus genitalis. After purification, each recombinant peptide eluted from a C18 column at a position closely matching its natural counterpart, strongly suggesting adoption of the native tertiary fold. Injection of purified recombinant peptides into blowflies (Lucilia cuprina) and crickets (Acheta domestica) revealed that two of the four recombinant peptides, named rDg3b and rDg12, inhibited escape behaviour in a manner that was rapid in onset (<1 min) and reversible. Homonuclear NMR solution structures revealed that rDg3b and rDg12 adopt cystine-stabilised α/ß defensin and inhibitor cystine knot folds, respectively. Although the closest known homologues of rDg3b at the level of primary structure are dipteran antimicrobial peptides such as sapecin and lucifensin, a DALI search showed that the tertiary structure of rDg3b most closely resembles the KV11.1-specific α-potassium channel toxin CnErg1 from venom of the scorpion Centruroides noxius. This is mainly due to the deletion of a large, unstructured loop between the first and second cysteine residues present in Dg3b homologues from non-asiloid, but not existing in asiloid, species. Patch-clamp electrophysiology experiments revealed that rDg3b shifts the voltage-dependence of KV11.1 channel activation to more depolarised potentials, but has no effect on KV1.3, KV2.1, KV10.1, KCa1.1, or the Drosophila Shaker channel. Although rDg12 shares the inhibitor cystine knot structure of many gating modifier toxins, rDg12 did not affect any of these KV channel subtypes. Our results demonstrate that multiple disulfide-rich peptide scaffolds have been convergently recruited into asilid and other animal venoms, and they provide insight into the molecular evolution accompanying their weaponisation.
中文翻译:

“即时”进行武器化:将结蛋白和防御素肽支架集中募集到掠夺性刺客果蝇的毒液中。
许多节肢动物毒液肽由于其特定的神经调节功能而具有作为生物杀虫剂,药物前导物和药理学工具的潜力。刺客蝇(Asilidae)是一种前掠角二倍体,可产生独特而复杂的富含肽的毒液,以杀死昆虫并阻止捕食者。然而,关于它们的毒液肽的结构和功能的了解很少。因此,我们使用了一种大肠杆菌周质表达系统来表达四个富含二硫键的肽,我们先前报道它们存在于巨大的刺客苍蝇Dolopus genitalis的毒液中。纯化后,每种重组肽都从C18色谱柱的与其天然对应物紧密匹配的位置洗脱下来,这强烈提示采用了天然三级折叠。将纯化的重组肽注射到苍蝇(Lucilia cuprina)和(Acheta domestica)中后发现,四种重组肽中的两个,分别名为rDg3b和rDg12,以快速起效(<1分钟)可逆的方式抑制了逃逸行为。同源核磁共振溶液结构表明,rDg3b和rDg12分别采用胱氨酸稳定的α/β防御素和抑制剂胱氨酸结折叠。尽管在一级结构水平上最接近的rDg3b同源物是二萜类抗菌肽,如赛普霉素和荧光素,但DALI搜索显示rDg3b的三级结构最类似于KV11.1特异性的来自毒液的α-钾通道毒素CnErg1蝎Centruroides noxius。这主要是由于删除了大量 Dg3b同源物中来自非等倍体的第一半胱氨酸残基和第二个半胱氨酸残基之间的非结构化环,但不存在于非淀粉样物质中。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的结果表明,多种富含二硫键的肽支架已被集中募集到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的结果表明,多种富含二硫键的肽支架已被集中募集到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。
更新日期:2019-12-21
中文翻译:

“即时”进行武器化:将结蛋白和防御素肽支架集中募集到掠夺性刺客果蝇的毒液中。
许多节肢动物毒液肽由于其特定的神经调节功能而具有作为生物杀虫剂,药物前导物和药理学工具的潜力。刺客蝇(Asilidae)是一种前掠角二倍体,可产生独特而复杂的富含肽的毒液,以杀死昆虫并阻止捕食者。然而,关于它们的毒液肽的结构和功能的了解很少。因此,我们使用了一种大肠杆菌周质表达系统来表达四个富含二硫键的肽,我们先前报道它们存在于巨大的刺客苍蝇Dolopus genitalis的毒液中。纯化后,每种重组肽都从C18色谱柱的与其天然对应物紧密匹配的位置洗脱下来,这强烈提示采用了天然三级折叠。将纯化的重组肽注射到苍蝇(Lucilia cuprina)和(Acheta domestica)中后发现,四种重组肽中的两个,分别名为rDg3b和rDg12,以快速起效(<1分钟)可逆的方式抑制了逃逸行为。同源核磁共振溶液结构表明,rDg3b和rDg12分别采用胱氨酸稳定的α/β防御素和抑制剂胱氨酸结折叠。尽管在一级结构水平上最接近的rDg3b同源物是二萜类抗菌肽,如赛普霉素和荧光素,但DALI搜索显示rDg3b的三级结构最类似于KV11.1特异性的来自毒液的α-钾通道毒素CnErg1蝎Centruroides noxius。这主要是由于删除了大量 Dg3b同源物中来自非等倍体的第一半胱氨酸残基和第二个半胱氨酸残基之间的非结构化环,但不存在于非淀粉样物质中。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的结果表明,多种富含二硫键的肽支架已被集中募集到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。膜片钳电生理实验表明,rDg3b将KV11.1通道激活的电压依赖性转移到更多去极化电位,但对KV1.3,KV2.1,KV10.1,KCa1.1或果蝇振荡器通道没有影响。 。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的结果表明,多种富含二硫键的肽支架已被集中募集到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。尽管rDg12具有许多门控修饰因子毒素的抑制剂胱氨酸结结构,但rDg12并不影响任何这些KV通道亚型。我们的研究结果表明,多种富含二硫键的肽支架已被集中招募到asilid和其他动物毒液中,它们为伴随武器化的分子进化提供了见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号