当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Randomized Placebo-Controlled Trial of Emricasan in Non-alcoholic Steatohepatitis (NASH) Cirrhosis with Severe Portal Hypertension
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.12.010 Guadalupe Garcia-Tsao 1 , Jaime Bosch 2 , Zeid Kayali 3 , Stephen A Harrison 4 , Manal F Abdelmalek 5 , Eric Lawitz 6 , Sanjaya K Satapathy 7 , Marwan Ghabril 8 , Mitchell L Shiffman 9 , Ziad H Younes 10 , Paul J Thuluvath 11 , Annalisa Berzigotti 12 , Agustin Albillos 13 , James M Robinson 14 , David T Hagerty 14 , Jean L Chan 14 , Arun J Sanyal 15 ,
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.12.010 Guadalupe Garcia-Tsao 1 , Jaime Bosch 2 , Zeid Kayali 3 , Stephen A Harrison 4 , Manal F Abdelmalek 5 , Eric Lawitz 6 , Sanjaya K Satapathy 7 , Marwan Ghabril 8 , Mitchell L Shiffman 9 , Ziad H Younes 10 , Paul J Thuluvath 11 , Annalisa Berzigotti 12 , Agustin Albillos 13 , James M Robinson 14 , David T Hagerty 14 , Jean L Chan 14 , Arun J Sanyal 15 ,
Affiliation
|
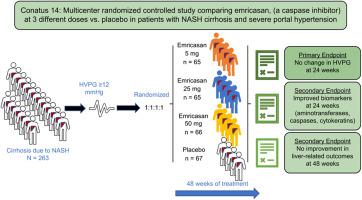
Background & Aims Emricasan, an oral pan-caspase inhibitor, decreased portal pressure in experimental cirrhosis and in an open-label study in patients with cirrhosis and severe portal hypertension, defined as a hepatic venous pressure gradient (HVPG) ≥12 mmHg. We aimed to confirm these results in a placebo-controlled study in patients with non-alcoholic steatohepatitis (NASH)-related cirrhosis. Methods We performed a multicenter double-blinded study, randomizing 263 patients with NASH-related cirrhosis and baseline HVPG ≥12 mmHg to twice daily oral emricasan 5 mg, 25 mg, 50 mg or placebo in a 1:1:1:1 ratio for up to 48 weeks. The primary endpoint was change in HVPG (ΔHVPG) at week 24. Secondary endpoints were changes in biomarkers (aminotransferases, caspases, cytokeratins) and development of liver-related outcomes. Results There were no significant differences in ΔHVPG for any emricasan dose vs. placebo (−0.21, −0.45, −0.58 mmHg, respectively) adjusted for baseline HVPG, compensation status, and non-selective beta-blocker use. Compensated patients (n = 201 [76%]) tended to have a greater decrease in HVPG (emricasan all vs. placebo, p = 0.06), the decrease being greater in those with higher baseline HVPG (p = 0.018), with a significant interaction between baseline HVPG (continuous, p = 0.024; dichotomous at 16 mmHg [median], p = 0.013) and treatment. Biomarkers decreased significantly with emricasan at week 24 but returned to baseline levels by week 48. New or worsening decompensating events (∼10% over median exposure of 337 days), progression in model for end-stage liver disease and Child-Pugh scores, and treatment-emergent adverse events were similar among treatment groups. Conclusions Despite a reduction in biomarkers indicating target engagement, emricasan was not associated with improvement in HVPG or clinical outcomes in patients with NASH-related cirrhosis and severe portal hypertension. Compensated patients with higher baseline HVPG had evidence of a small treatment effect. Emricasan treatment appeared safe and well-tolerated. Lay summary Cirrhosis (scarring of the liver) is the main consequence of non-alcoholic steatohepatitis (NASH). Cirrhosis leads to high pressure in the portal vein which accounts for most of the complications of cirrhosis. Reducing portal pressure is beneficial in patients with cirrhosis. We studied the possibility that emricasan, a drug that improves inflammation and scarring in the liver, would reduce portal pressure in patients with NASH-related cirrhosis and severe portal hypertension. Our results in a large, prospective, double-blind study could not demonstrate a beneficial effect of emricasan in these patients. Clinical Trial Number Clinical Trials.gov #NCT02960204.
中文翻译:

Emricasan 在非酒精性脂肪性肝炎 (NASH) 肝硬化伴严重门静脉高压患者中的随机安慰剂对照试验
背景与目的 Emricasan 是一种口服泛半胱天冬酶抑制剂,可降低实验性肝硬化和肝硬化和严重门静脉高压(定义为肝静脉压力梯度 (HVPG) ≥ 12 mmHg)患者的开放标签研究中的门静脉压力。我们旨在在一项针对非酒精性脂肪性肝炎 (NASH) 相关肝硬化患者的安慰剂对照研究中证实这些结果。方法 我们进行了一项多中心双盲研究,将 263 名 NASH 相关肝硬化患者和基线 HVPG ≥ 12 mmHg 随机分配至每天两次口服 emricasan 5 mg、25 mg、50 mg 或安慰剂,比例为 1:1:1:1长达 48 周。主要终点是第 24 周 HVPG (ΔHVPG) 的变化。次要终点是生物标志物(转氨酶、半胱天冬酶、细胞角蛋白)的变化和肝脏相关结果的发展。结果 任何 emricasan 剂量与安慰剂的 ΔHVPG 均无显着差异(分别为 -0.21、-0.45、-0.58 mmHg)针对基线 HVPG、代偿状态和非选择性 β 受体阻滞剂使用进行调整。代偿患者(n = 201 [76%])的 HVPG 降低幅度更大(emricasan all vs.安慰剂,p = 0.06),基线 HVPG 较高的患者(p = 0.018)降低幅度更大,显着基线 HVPG(连续,p = 0.024;在 16 mmHg 处二分法 [中值],p = 0.013)和治疗之间的相互作用。使用 emricasan 时,生物标志物在第 24 周显着降低,但在第 48 周时恢复到基线水平。治疗组间出现的不良事件和治疗中出现的不良事件相似。结论 尽管指示靶标参与的生物标志物减少,但 emricasan 与 NASH 相关肝硬化和严重门静脉高压患者的 HVPG 或临床结果的改善无关。具有较高基线 HVPG 的代偿患者有证据表明治疗效果很小。Emricasan 治疗似乎安全且耐受性良好。总结 肝硬化(肝脏瘢痕形成)是非酒精性脂肪性肝炎 (NASH) 的主要后果。肝硬化导致门静脉高压,这是大部分肝硬化并发症的原因。降低门静脉压力对肝硬化患者有益。我们研究了 emricasan 这种改善肝脏炎症和疤痕的药物的可能性 将降低 NASH 相关肝硬化和严重门静脉高压患者的门静脉压力。我们在一项大型、前瞻性、双盲研究中的结果无法证明 emricasan 对这些患者的有益作用。临床试验编号 Clinical Trials.gov #NCT02960204。
更新日期:2020-05-01
中文翻译:

Emricasan 在非酒精性脂肪性肝炎 (NASH) 肝硬化伴严重门静脉高压患者中的随机安慰剂对照试验
背景与目的 Emricasan 是一种口服泛半胱天冬酶抑制剂,可降低实验性肝硬化和肝硬化和严重门静脉高压(定义为肝静脉压力梯度 (HVPG) ≥ 12 mmHg)患者的开放标签研究中的门静脉压力。我们旨在在一项针对非酒精性脂肪性肝炎 (NASH) 相关肝硬化患者的安慰剂对照研究中证实这些结果。方法 我们进行了一项多中心双盲研究,将 263 名 NASH 相关肝硬化患者和基线 HVPG ≥ 12 mmHg 随机分配至每天两次口服 emricasan 5 mg、25 mg、50 mg 或安慰剂,比例为 1:1:1:1长达 48 周。主要终点是第 24 周 HVPG (ΔHVPG) 的变化。次要终点是生物标志物(转氨酶、半胱天冬酶、细胞角蛋白)的变化和肝脏相关结果的发展。结果 任何 emricasan 剂量与安慰剂的 ΔHVPG 均无显着差异(分别为 -0.21、-0.45、-0.58 mmHg)针对基线 HVPG、代偿状态和非选择性 β 受体阻滞剂使用进行调整。代偿患者(n = 201 [76%])的 HVPG 降低幅度更大(emricasan all vs.安慰剂,p = 0.06),基线 HVPG 较高的患者(p = 0.018)降低幅度更大,显着基线 HVPG(连续,p = 0.024;在 16 mmHg 处二分法 [中值],p = 0.013)和治疗之间的相互作用。使用 emricasan 时,生物标志物在第 24 周显着降低,但在第 48 周时恢复到基线水平。治疗组间出现的不良事件和治疗中出现的不良事件相似。结论 尽管指示靶标参与的生物标志物减少,但 emricasan 与 NASH 相关肝硬化和严重门静脉高压患者的 HVPG 或临床结果的改善无关。具有较高基线 HVPG 的代偿患者有证据表明治疗效果很小。Emricasan 治疗似乎安全且耐受性良好。总结 肝硬化(肝脏瘢痕形成)是非酒精性脂肪性肝炎 (NASH) 的主要后果。肝硬化导致门静脉高压,这是大部分肝硬化并发症的原因。降低门静脉压力对肝硬化患者有益。我们研究了 emricasan 这种改善肝脏炎症和疤痕的药物的可能性 将降低 NASH 相关肝硬化和严重门静脉高压患者的门静脉压力。我们在一项大型、前瞻性、双盲研究中的结果无法证明 emricasan 对这些患者的有益作用。临床试验编号 Clinical Trials.gov #NCT02960204。











































 京公网安备 11010802027423号
京公网安备 11010802027423号