Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.39013 Chaohua Liu 1 , Qiaoyan Yang 1 , Qian Zhu 2 , Xiaopeng Lu 2 , Meiting Li 1 , Tianyun Hou 1 , Zhiming Li 2 , Ming Tang 2 , Yinglu Li 2 , Hui Wang 1, 2 , Yang Yang 1 , Haiying Wang 1 , Ying Zhao 1 , He Wen 2 , Xiangyu Liu 2 , Zebin Mao 1 , Wei-Guo Zhu 1, 2
|
Background and Aim: DOT1L regulates various genes involved in cancer onset and progression by catalyzing H3K79 methylation, but how DOT1L activity itself is regulated is unclear. Here, we aimed to identify specific DOT1L post-translational modifications that might regulate DOT1L activity and thus impact on colorectal cancer (CRC) progression.
Methods: We conducted affinity purification and mass spectrometry to explore DOT1L post-translational modifications. We then established transwell migration and invasion assays to specifically investigate the role of DOT1L(K358) acetylation on CRC cellular behavior in vitro and a bioluminescence imaging approach to determine the role of DOT1L(K358) acetylation in CRC metastasis in vivo. We performed chromatin immunoprecipitation to identify DOT1L acetylation-controlled target genes. Finally, we used immunohistochemical staining of human tissue arrays to examine the relevance of DOT1L(K358) acetylation in CRC progression and metastasis and the correlation between DOT1L acetylation and CBP.
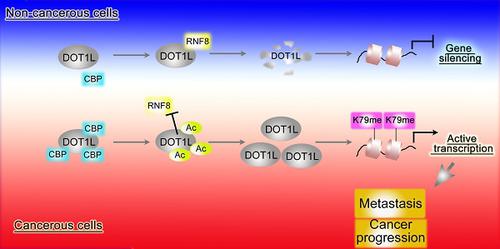
Results: We found that CBP mediates DOT1L K358 acetylation in human colon cancer cells and positively correlates with CRC stages. Mechanistically, DOT1L acetylation confers DOT1L stability by preventing the binding of RNF8 to DOT1L and subsequent proteasomal degradation, but does not affect its enzyme activity. Once stabilized, DOT1L can catalyze the H3K79 methylation of genes involved in epithelial-mesenchymal transition, including SNAIL and ZEB1. An acetylation mimic DOT1L mutant (Q358) could induce a cancer-like phenotype in vitro, characterized by metastasis and invasion. Finally, DOT1L(K358) acetylation correlated with CRC progression and a poor survival rate as well as with high CBP expression.
Conclusions: DOT1L acetylation by CBP drives CRC progression and metastasis. Targeting DOT1L deacetylation signaling is a potential therapeutic strategy for DOT1L-driven cancers.
中文翻译:

CBP介导的DOT1L乙酰化赋予DOT1L稳定性并促进癌症转移。
背景与目的:DOT1L通过催化H3K79甲基化来调节与癌症发作和进展有关的各种基因,但是尚不清楚DOT1L活性本身是如何被调节的。在这里,我们旨在确定特定的DOT1L翻译后修饰,这些修饰可能会调节DOT1L的活性,从而影响结直肠癌(CRC)的进展。
方法:我们进行了亲和纯化和质谱分析,以探讨DOT1L的翻译后修饰。然后,我们建立了跨孔迁移和侵袭试验,以专门研究DOT1L(K358)乙酰化在体外CRC细胞行为中的作用,并采用生物发光成像方法确定DOT1L(K358)乙酰化在体内CRC转移中的作用。我们进行了染色质免疫沉淀,以鉴定DOT1L乙酰化控制的靶基因。最后,我们使用了人体组织阵列的免疫组织化学染色来检查DOT1L(K358)乙酰化在CRC进程和转移中的相关性以及DOT1L乙酰化与CBP之间的相关性。
结果:我们发现CBP在人结肠癌细胞中介导了DOT1L K358乙酰化,并且与CRC分期呈正相关。从机理上讲,DOT1L乙酰化通过防止RNF8与DOT1L结合以及随后的蛋白酶体降解而赋予DOT1L稳定性,但不影响其酶活性。一旦稳定,DOT1L可以催化涉及上皮-间质转化的基因(包括SNAIL和ZEB1)的H3K79甲基化。乙酰化模拟DOT1L突变体(Q358)可以在体外诱导癌样表型,其特征是转移和侵袭。最后,DOT1L(K358)乙酰化与CRC进展,不良的生存率以及高CBP表达相关。
结论:CBP对DOT1L的乙酰化作用可促进CRC的进展和转移。靶向DOT1L脱乙酰信号是DOT1L驱动的癌症的潜在治疗策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号