Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.41309 Lu Liu 1, 2 , Huseyin Karagoz 3 , Michele Herneisey 1, 2 , Fatih Zor 3 , Takaaki Komatsu 4 , Shannon Loftus 1, 2 , Bratislav M Janjic 5 , Vijay S Gorantla 3 , Jelena M Janjic 1, 2
|
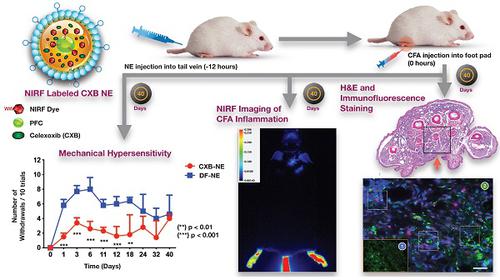
Monocyte derived macrophages (MDMs) infiltrate sites of infection or injury and upregulate cyclooxygenase-2 (COX-2), an enzyme that stimulates prostaglandin-E2 (PgE2). Nanotheranostics combine therapeutic and diagnostic agents into a single nanosystem. In previous studies, we demonstrated that a nanotheranostic strategy, based on theranostic nanoemulsions (NE) loaded with a COX-2 inhibitor (celecoxib, CXB) and equipped with near-infrared fluorescent (NIRF) reporters, can specifically target circulating monocytes and MDMs. The anti-inflammatory and anti-nociceptive effects of such cell-specific COX-2 inhibition lasted several days following Complete Freund's Adjuvant (CFA) or nerve injury in male mice. The overall goal of this study was to investigate the extended (up to 40 days) impact of MDM-targeted COX-2 inhibition and any sex-based differences in treatment response; both of which remain unknown. Our study also evaluates the feasibility and efficacy of a preclinical nanotheranostic strategy for mechanistic investigation of the impact of such sex differences on clinical outcomes.
Methods: CFA was administered into the right hind paws of male and female mice. All mice received a single intravenous dose of NIRF labeled CXB loaded NE twelve hours prior to CFA injection. In vivo whole body NIRF imaging and mechanical hypersensitivity assays were performed sequentially and ex vivo NIRF imaging and immunohistopathology of foot pad tissues were performed at the end point of 40 days.
Results: Targeted COX-2 inhibition of MDMs in male and female mice successfully improved mechanical hypersensitivity after CFA injury. However, we observed distinct sex-specific differences in the intensity or longevity of the nociceptive responses. In males, a single dose of CXB-NE administered via tail vein injection produced significant improved mechanical hypersensitivity for 32 days as compared to the drug free NE (DF-NE) (untreated) control group. In females, CXB-NE produced similar, though less prominent and shorter-lived effects, lasting up to 11 days. NIRF imaging confirmed that CXB-NE can be detected up to day 40 in the CFA injected foot pad tissues of both sexes. There were distinct signal distribution trends between males and females, suggesting differences in macrophage infiltration dynamics between the sexes. This may also relate to differences in macrophage turnover rate between the sexes, a possibility that requires further investigation in this model.
Conclusions: For the first time, this study provides unique insight into MDM dynamics and the early as well as longer-term targeted effects and efficacy of a clinically translatable nanotheranostic agent on MDM mediated inflammation. Our data supports the potential of nanotheranostics as presented in elucidating the kinetics, dynamics and sex-based differences in the adaptive or innate immune responses to inflammatory triggers. Taken together, our study findings lead us closer to true personalized, sex-specific pain nanomedicine for a wide range of inflammatory diseases.
中文翻译:

在以巨噬细胞为靶点的纳米治疗的小鼠CFA炎症模型中发现的性别差异。
单核细胞衍生的巨噬细胞(MDM)渗透到感染或损伤部位,并上调环氧合酶2(COX-2),一种刺激前列腺素E2(PgE2)的酶。纳米疗法将治疗剂和诊断剂组合到一个纳米系统中。在以前的研究中,我们证明了基于治疗性纳米乳剂(NE)的纳米治疗策略,该治疗剂中装有COX-2抑制剂(celecoxib,CXB)并配备了近红外荧光(NIRF)报道分子,可以特异性靶向循环单核细胞和MDM。在完全弗氏佐剂(CFA)或雄性小鼠神经损伤后,这种细胞特异性COX-2抑制作用的抗炎和抗伤害作用持续了几天。这项研究的总体目标是调查针对MDM的COX-2抑制作用的延长(最多40天)影响以及治疗反应中任何基于性别的差异。两者仍然未知。我们的研究还评估了临床前纳米治疗策略对这种性别差异对临床结果的影响进行机械研究的可行性和有效性。
方法:将CFA施用于雄性和雌性小鼠的右后爪。在CFA注射前十二小时,所有小鼠接受单次静脉内剂量的NIRF标记的CXB静脉内负荷NE。依次进行体内全身NIRF成像和机械性超敏反应测定,并在40天结束时进行足垫组织的离体NIRF成像和免疫组织病理学检查。
结果:雄性和雌性小鼠对MDM的靶向COX-2抑制成功改善了CFA损伤后的机械性超敏反应。但是,我们观察到伤害反应的强度或寿命存在明显的性别差异。在男性中,与无药物的NE(DF-NE)(未治疗)对照组相比,通过尾静脉注射单剂量的CXB-NE在32天内产生了显着改善的机械超敏性。在女性中,CXB-NE产生了相似但不明显且寿命较短的效果,可持续长达11天。NIRF成像证实,直至第40天,在男女CFA注射的脚垫组织中都可以检测到CXB-NE。男性和女性之间存在明显的信号分布趋势,表明性别之间巨噬细胞浸润动力学的差异。
结论:这项研究首次为MDM动力学以及临床可翻译的纳米治疗剂对MDM介导的炎症的早期及长期靶向作用和疗效提供了独特的见解。我们的数据支持纳米治疗的潜力,阐明了对炎症触发因素的适应性或先天性免疫反应的动力学,动力学和基于性别的差异。综上所述,我们的研究发现使我们更接近针对各种炎症性疾病的真正个性化,针对性别的疼痛纳米医学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号