当前位置:
X-MOL 学术
›
J. Environ. Manag.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of the diuretic hydrochlorothiazide by UV/Solar radiation assisted oxidation processes.
Journal of Environmental Management ( IF 8.0 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.jenvman.2019.109973 M Fernández-Perales 1 , M Sánchez-Polo 1 , M Rozalen 1 , M V López-Ramón 2 , A J Mota 1 , J Rivera-Utrilla 1
Journal of Environmental Management ( IF 8.0 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.jenvman.2019.109973 M Fernández-Perales 1 , M Sánchez-Polo 1 , M Rozalen 1 , M V López-Ramón 2 , A J Mota 1 , J Rivera-Utrilla 1
Affiliation
|
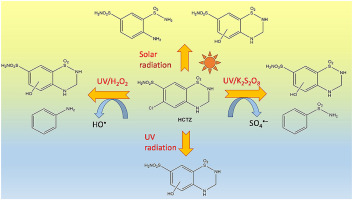
The objective of this study was to analyse the effectiveness of advanced oxidation processes (AOPs) with Solar and UV radiation (UV/H2O2, UV/K2S2O8) for the degradation of hydrochlorothiazide (HCTZ), a widely used diuretic drug, in aqueous solution focusing on the influence of four experimental parameters: initial concentration of HCTZ, solution pH, nature of the water matrix, and initial concentration of radicals. The obtained results showed that using both kinds of direct photolysis (UV and Solar), the percentage of degraded HCTZ was low, but there was a decrease in the degradation rate favored by the increase of the initial concentration of this pollutant. In addition, the degradation rates were higher at acid pHs. With regard to the nature of water, the degradation rate varied in the order: ultrapure > superficial > tap water. This is due to the presence of organic and inorganic matter (bicarbonates, nitrates, and chlorides) in surface and tap water, that react with the radicals generated, which reduces the availability of radical species, generating competitive kinetics. The presence of radical-promoter species increased the degradation rate of the pollutant, reaching a degradation of 100% of HCTZ after 20 min of treatment. The results obtained point out that the degradation rate was higher in the presence of HO radicals. This behavior was attributed to the higher oxidation power of HO versus radicals. The determination of the degradation by-products led to structures very similar to the parent compound. For example, the corresponding hydroxylated dechlorinated derivative of HCTZ was found in all the systems used. The cytotoxicity test showed that these byproducts have a lower toxicity than the original product. Finally, the economic viability study confirmed that the UV/K2S2O8 system has the lowest cost.
中文翻译:

紫外线/太阳能辅助氧化工艺可降解利尿剂氢氯噻嗪。
这项研究的目的是分析利用太阳和紫外线辐射(UV / H2O2,UV / K2S2O8)进行的高级氧化过程(AOP)在水溶液聚焦中对利尿剂氢氯噻嗪(HCTZ)的降解的有效性。四个实验参数的影响:HCTZ的初始浓度,溶液的pH值,水基质的性质以及自由基的初始浓度。所得结果表明,使用两种直接光解法(UV和太阳能),降解的HCTZ的百分比均较低,但由于该污染物初始浓度的增加,降解速率有所降低。另外,在酸性pH下降解速率较高。关于水的性质,降解速率按以下顺序变化:超纯>表层>自来水。这是由于在地表水和自来水中存在有机和无机物质(碳酸氢盐,硝酸盐和氯化物),它们与生成的自由基反应,从而降低了自由基种类的可用性,从而产生了竞争动力学。自由基促进剂的存在增加了污染物的降解率,处理20分钟后,HCTZ的降解率达到了100%。所得结果指出,在存在HO自由基的情况下,降解速率较高。此行为归因于HO与自由基相比具有更高的氧化能力。降解副产物的测定导致与母体化合物非常相似的结构。例如,在所有使用的系统中均发现了相应的HCTZ羟基化脱氯衍生物。细胞毒性测试表明,这些副产物的毒性低于原始产物。最后,经济可行性研究证实,UV / K2S2O8系统具有最低的成本。
更新日期:2019-12-20
中文翻译:

紫外线/太阳能辅助氧化工艺可降解利尿剂氢氯噻嗪。
这项研究的目的是分析利用太阳和紫外线辐射(UV / H2O2,UV / K2S2O8)进行的高级氧化过程(AOP)在水溶液聚焦中对利尿剂氢氯噻嗪(HCTZ)的降解的有效性。四个实验参数的影响:HCTZ的初始浓度,溶液的pH值,水基质的性质以及自由基的初始浓度。所得结果表明,使用两种直接光解法(UV和太阳能),降解的HCTZ的百分比均较低,但由于该污染物初始浓度的增加,降解速率有所降低。另外,在酸性pH下降解速率较高。关于水的性质,降解速率按以下顺序变化:超纯>表层>自来水。这是由于在地表水和自来水中存在有机和无机物质(碳酸氢盐,硝酸盐和氯化物),它们与生成的自由基反应,从而降低了自由基种类的可用性,从而产生了竞争动力学。自由基促进剂的存在增加了污染物的降解率,处理20分钟后,HCTZ的降解率达到了100%。所得结果指出,在存在HO自由基的情况下,降解速率较高。此行为归因于HO与自由基相比具有更高的氧化能力。降解副产物的测定导致与母体化合物非常相似的结构。例如,在所有使用的系统中均发现了相应的HCTZ羟基化脱氯衍生物。细胞毒性测试表明,这些副产物的毒性低于原始产物。最后,经济可行性研究证实,UV / K2S2O8系统具有最低的成本。










































 京公网安备 11010802027423号
京公网安备 11010802027423号