Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.jorganchem.2019.121087 Pushpanathan N. Sathishkumar , Padinhattath Sachind Prabha , Nattamai S.P. Bhuvanesh , Ramasamy Karvembu
|
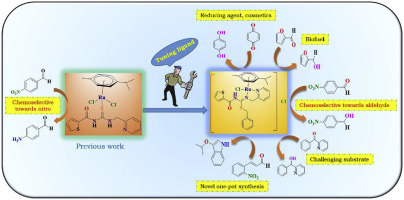
Ru(II)-p-cymene complexes (1–3) containing picolyl based pseudo-acylthiourea ligands (L1-L3) were synthesized and characterized. The crystallographic study confirmed the molecular structures of all the ligands (L1-L3) and complex 3. The catalytic activity of the complexes was tested mainly towards TH of carbonyl compounds and nitroarenes. The influence of steric and electronic effects of the ligands on the chemoselectivity and reactivity were reported. The catalytic activity was enhanced and chemoselectivity was switched after tuning the ligands in the catalysts, compared to their corresponding unmodified Ru(II)-p-cymene complexes. The catalysis was extended to a broad range of substrates including some challenging systems like furfural, benzoylpyridine, benzoquinone, chromanone, etc. The strategy of tuning the bifunctional ligands in the catalysts for effective and selective catalysis worked nicely. Further, the catalysis was extended to one pot synthesis of 3-isopropoxyindole from 2-nitrocinnamaldehyde, the first synthetic route similar to Baeyer Emmerling indole synthesis. All the catalytic experiments exhibited high conversion and selectivity.
中文翻译:

调节Ru(II)催化剂中的酰基硫脲配体,以改变转移氢化反应的反应性和化学选择性,并通过新的合成方法合成3-异丙氧基-1H-吲哚
的Ru(II) - p -cymene复合物(1 - 3)含基于吡啶甲基伪酰基硫脲配体(L 1 -L 3)的合成和表征。结晶研究证实所有的配体(L的分子结构1 -L 3)和复杂的3复合物的催化活性,主要是测试朝TH羰基化合物和硝基芳烃的。报道了配体的空间和电子效应对化学选择性和反应性的影响。与相应的未修饰Ru(II)-p相比,在调节催化剂中的配体后,催化活性得到了增强,化学选择性也得到了改变-cymene复合物。催化作用扩展到了广泛的底物,包括一些具有挑战性的系统,例如糠醛,苯甲酰吡啶,苯醌,苯并二氢吡喃酮等。为有效和选择性催化而调节催化剂中双功能配体的策略效果很好。此外,该催化作用扩展到由2-硝基肉桂醛一锅合成3-异丙氧基吲哚,这是类似于Baeyer Emmerling吲哚合成的第一个合成路线。所有的催化实验均显示出高转化率和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号