当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of surface functionalized hydroxyapatite nanoparticles for enhanced specificity towards tumor cells.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.ejps.2019.105206 Gunjan Verma 1 , Neena G Shetake 2 , Shruti Pandrekar 3 , B N Pandey 2 , P A Hassan 1 , K I Priyadarsini 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.ejps.2019.105206 Gunjan Verma 1 , Neena G Shetake 2 , Shruti Pandrekar 3 , B N Pandey 2 , P A Hassan 1 , K I Priyadarsini 1
Affiliation
|
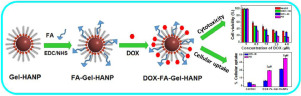
Nanoparticles coupled with targeting moieties have attracted a great deal of attention for cancer therapy since they can facilitate site-specific delivery of drug and significantly limit the side effects of systemic chemotherapy. In this study, our aim is to develop surface functionalized hydroxyapatite nanoparticles, which could provide binding sites for a cancer cell targeting ligand, folic acid (FA) as well as an anticancer drug, doxorubicin hydrochloride (DOX). In order to attain dual functionalities, hydroxyapatite nanoparticles were functionalized with gelatin molecules. Gelatin, being a protein has both carboxyl and amine moieties, which makes it suitable for binding of DOX and FA. FA was chemically conjugated to the nanoparticles through an EDCNHS coupling reaction. The formation of single-phase hydroxyapatite nanostructure was ascertained by X-ray diffraction studies and the presence of organic moieties on the surface of nanoparticles was evident from Fourier transform infrared spectroscopy, thermogravimetric analysis and U.V.-visible spectroscopy. The FA-conjugated nanoparticles (FA-Gel-HANPs) showed high affinity towards DOX and pH-responsive sustained release of drug with higher release rate under acidic pH conditions, desired for cancer therapy. The FA-Gel-HANPs showed negligible cytotoxicity towards different cell lines (HepG2, WEHI-164, KB, WI-26 VA4 and WRL-68). However, DOX loaded nanoparticles (DOX-FA-Gel-HANPs) exhibited significant toxicity towards these cells, which was however highest in folate receptor (FR)-overexpressing, KB cells. These results were correlated with enhanced cellular uptake of DOX-FA-Gel-HANPs in KB cells in comparison to FR-deficient, WRL-68 cells studied by confocal laser scanning microscopy and flow cytometry. Moreover, cell cycle analysis in KB cells, showed higher sub-G1 population, indicating apoptosis as one of the cell death mechanisms. Overall, this study suggests that DOX-FA-Gel-HANPs could serve as a promising tumor-targeted drug delivery system.
中文翻译:

用于增强对肿瘤细胞特异性的表面功能化羟基磷灰石纳米粒子的开发。
纳米粒子与靶向部分相结合已经引起了癌症治疗的广泛关注,因为它们可以促进药物的定点递送并显着限制全身化学疗法的副作用。在这项研究中,我们的目标是开发表面功能化的羟基磷灰石纳米粒子,该纳米粒子可以为靶向癌细胞的配体叶酸(FA)以及抗癌药物盐酸阿霉素(DOX)提供结合位点。为了获得双重功能,用明胶分子将羟基磷灰石纳米颗粒功能化。明胶是一种具有羧基和胺基的蛋白质,这使其适用于DOX和FA的结合。FA通过EDCNHS偶联反应化学偶联到纳米颗粒上。通过X射线衍射研究确定了单相羟基磷灰石纳米结构的形成,并且通过傅立叶变换红外光谱,热重分析和UV-可见光谱可明显看出纳米颗粒表面上有机部分的存在。与FA结合的纳米颗粒(FA-Gel-HANPs)对DOX具有高亲和力,并且在酸性pH条件下具有更高的释放速率,对药物的pH响应持续释放具有较高的释放速度,这是癌症治疗所需要的。FA-Gel-HANPs对不同细胞系(HepG2,WEHI-164,KB,WI-26 VA4和WRL-68)的细胞毒性可忽略不计。但是,载有DOX的纳米颗粒(DOX-FA-Gel-HANPs)对这些细胞表现出显着的毒性,但是在过量表达叶酸受体(FR)的KB细胞中毒性最高。与通过共聚焦激光扫描显微镜和流式细胞术研究的缺乏FR的WRL-68细胞相比,这些结果与KB细胞中DOX-FA-Gel-HANPs的细胞摄取增强有关。此外,KB细胞的细胞周期分析显示,sub-G1种群较高,表明细胞凋亡是细胞死亡的机制之一。总体而言,这项研究表明,DOX-FA-Gel-HANPs可以作为有希望的靶向肿瘤的药物递送系统。
更新日期:2019-12-20
中文翻译:

用于增强对肿瘤细胞特异性的表面功能化羟基磷灰石纳米粒子的开发。
纳米粒子与靶向部分相结合已经引起了癌症治疗的广泛关注,因为它们可以促进药物的定点递送并显着限制全身化学疗法的副作用。在这项研究中,我们的目标是开发表面功能化的羟基磷灰石纳米粒子,该纳米粒子可以为靶向癌细胞的配体叶酸(FA)以及抗癌药物盐酸阿霉素(DOX)提供结合位点。为了获得双重功能,用明胶分子将羟基磷灰石纳米颗粒功能化。明胶是一种具有羧基和胺基的蛋白质,这使其适用于DOX和FA的结合。FA通过EDCNHS偶联反应化学偶联到纳米颗粒上。通过X射线衍射研究确定了单相羟基磷灰石纳米结构的形成,并且通过傅立叶变换红外光谱,热重分析和UV-可见光谱可明显看出纳米颗粒表面上有机部分的存在。与FA结合的纳米颗粒(FA-Gel-HANPs)对DOX具有高亲和力,并且在酸性pH条件下具有更高的释放速率,对药物的pH响应持续释放具有较高的释放速度,这是癌症治疗所需要的。FA-Gel-HANPs对不同细胞系(HepG2,WEHI-164,KB,WI-26 VA4和WRL-68)的细胞毒性可忽略不计。但是,载有DOX的纳米颗粒(DOX-FA-Gel-HANPs)对这些细胞表现出显着的毒性,但是在过量表达叶酸受体(FR)的KB细胞中毒性最高。与通过共聚焦激光扫描显微镜和流式细胞术研究的缺乏FR的WRL-68细胞相比,这些结果与KB细胞中DOX-FA-Gel-HANPs的细胞摄取增强有关。此外,KB细胞的细胞周期分析显示,sub-G1种群较高,表明细胞凋亡是细胞死亡的机制之一。总体而言,这项研究表明,DOX-FA-Gel-HANPs可以作为有希望的靶向肿瘤的药物递送系统。










































 京公网安备 11010802027423号
京公网安备 11010802027423号