Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.tetlet.2019.151553 Kseniya V. Belyaeva , Lina P. Nikitina , Andrei V. Afonin , Alexander V. Vashchenko , Boris A. Trofimov
|
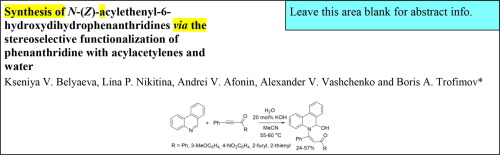
The facile (55-60 oC) reductive functionalization of phenanthridine with acylacetylenes and water in MeCN led to the stereoselective formation of N-(Z)-acylethenyl-6-hydroxydihydrophenanthridines in 24-57% yield. This functionalization contrasts with the reactions of pyridines and quinolines with the same reagents wherein either ring opening (for pyridines) or diverse functionalizations (for quinolines) of the azine ring occurs. The functionalized dihydrophenanthridines thus obtained represent a new prospective family of precursors and building blocks for biochemistry and drug discovery.
中文翻译:

通过菲啶与酰基乙炔和水的立体选择性官能化合成N-(Z)-丙烯基-6-羟基二氢菲啶
在MeCN中用乙酰乙炔和水对菲啶进行简便的(55-60 o C)还原功能化,导致N-(Z)-酰基乙烯基-6-羟基二氢菲啶的立体选择性形成,产率为24-57%。该官能化与吡啶和喹啉与相同试剂的反应形成对比,在相同的试剂中,发生了嗪环的开环(对于吡啶)或多种官能化(对于喹啉)。如此获得的官能化二氢菲啶代表了生物化学和药物发现的前体和构建基的新的预期家族。











































 京公网安备 11010802027423号
京公网安备 11010802027423号