当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to Functionalized Pyrrolidones via Copper-Catalyzed Aminooxygenation of Unactivated Alkenes
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.tetlet.2019.151540 Wei-Hao Rao , Li-Li Jiang , Fang-Yuan Chen , Meng Zhang , Yi-Yuan Guo , Jia-Jia Chen , Ying Cui , Guo-Dong Zou , Lin Tang
中文翻译:

通过铜催化的未活化烯烃的氨基氧化作用获得功能化的吡咯烷酮
更新日期:2019-12-20
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.tetlet.2019.151540 Wei-Hao Rao , Li-Li Jiang , Fang-Yuan Chen , Meng Zhang , Yi-Yuan Guo , Jia-Jia Chen , Ying Cui , Guo-Dong Zou , Lin Tang
|
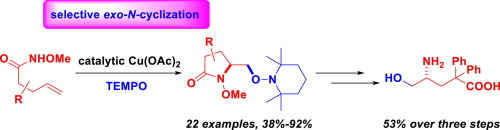
A simple and efficient copper-catalyzed aminooxygenation of unactivated alkenes with commercially available (2,2,6,6-tetramethylpiperidin-1-yl)-oxyl (TEMPO) as an oxygen source is reported. With this protocol, a variety of functionalized pyrrolidones were obtained in good to excellent yields, and good tolerance of functional groups and broad substrate scope were observed. Notably, the N-cyclization step selectively proceeded in an exo manner, and this protocol provides an efficient and straightforward approach to prepare vicinal amino alcohols.
中文翻译:

通过铜催化的未活化烯烃的氨基氧化作用获得功能化的吡咯烷酮
据报道,以市售的(2,2,6,6-四甲基哌啶-1-基)-氧基(TEMPO)作为氧源,对未活化的烯烃进行了简单而有效的铜催化的氨基氧化。通过该协议,可以以良好的产率获得各种功能化的吡咯烷酮,并观察到对官能团的良好耐受性和广泛的底物范围。值得注意的是,N-环化步骤以外切方式选择性地进行,并且该方案提供了制备邻位氨基醇的有效且直接的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号