当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A DFT study on mechanisms of CO2 coupling with propargylic alcohols using alkali carbonates
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-20 , DOI: 10.1002/qua.26150 Weiyi Li 1 , Geng Leng 2 , Caiqin Li 3 , Yajing Lyu 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-20 , DOI: 10.1002/qua.26150 Weiyi Li 1 , Geng Leng 2 , Caiqin Li 3 , Yajing Lyu 1
Affiliation
|
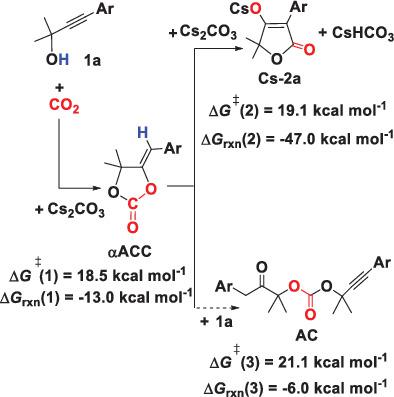
The mechanisms of CO2 coupling with the propargylic alcohol using alkali carbonates M2CO3 (M = Li, Na, K, Cs) have been investigated by means of density functional theory calculations. The calculations reveal that the target product tetronic acid (TA) is yielded through two stages: (a) the formation of the α‐alkylidene cyclic carbonate (αACC) intermediate via Cs2CO3‐mediated carboxylative cyclization of the propargylic alcohol with CO2, and (b) the conversion of the αACC intermediate with Cs2CO3 to produce the cesium salt of the TA. Since the overall kinetic barriers for the two stages are comparable and affordable, the excellent chemoselectivity to the TA should be primarily originated from the high thermodynamic stability of the cesium salt of the TA. Moreover, relative to the TA, the possibility to yield the by‐product acyclic carbonate can be excluded due to the both kinetics and thermodynamic inferiority. This result is different from the organic base‐mediated reaction. Alternatively, our calculations predict that CsHCO3 together generated with the cesium salt of the TA might also be an available mediating reagent for the incorporation of CO2 with the propargylic alcohol. Compared to other alkali carbonates M2CO3 (M = Li, Na, K), the stronger basicity of Cs2CO3 and the lower ionic potential of cesium ion can raise the effective concentration of the αACC intermediate, and thus the conversion of the αACC intermediate into the cesium salt of the TA can be achieved with high yield.
中文翻译:

DFT研究碱式碳酸盐将CO2与炔丙醇偶联的机理
通过密度泛函理论计算,研究了使用碱金属碳酸盐M 2 CO 3(M = Li,Na,K,Cs)使CO 2与炔丙醇偶联的机理。计算结果表明,目标产物tetronic酸(TA)分为两个阶段:(a)通过Cs 2 CO 3介导的炔丙醇与CO 2的羧基环化反应形成α-亚烷基环状碳酸酯(αACC)中间体(b)αACC中间体与Cs 2 CO 3的转化产生TA的铯盐。由于这两个阶段的整体动力学障碍是可比较的且价格合理,因此对TA的出色的化学选择性应主要源自TA铯盐的高热力学稳定性。此外,相对于TA,由于动力学和热力学劣势,可以排除产生副产物无环碳酸酯的可能性。该结果不同于有机碱介导的反应。或者,我们的计算预测,与TA的铯盐一起生成的CsHCO 3可能也是将CO 2与炔丙醇结合的一种可用的介导试剂。与其他碱金属碳酸盐M 2 CO 3相比(M = Li,Na,K),Cs 2 CO 3的强碱性和铯离子的较低离子电势可以提高αACC中间体的有效浓度,从而将αACC中间体转化为铯的铯盐。可以高收率获得TA。
更新日期:2020-03-02
中文翻译:

DFT研究碱式碳酸盐将CO2与炔丙醇偶联的机理
通过密度泛函理论计算,研究了使用碱金属碳酸盐M 2 CO 3(M = Li,Na,K,Cs)使CO 2与炔丙醇偶联的机理。计算结果表明,目标产物tetronic酸(TA)分为两个阶段:(a)通过Cs 2 CO 3介导的炔丙醇与CO 2的羧基环化反应形成α-亚烷基环状碳酸酯(αACC)中间体(b)αACC中间体与Cs 2 CO 3的转化产生TA的铯盐。由于这两个阶段的整体动力学障碍是可比较的且价格合理,因此对TA的出色的化学选择性应主要源自TA铯盐的高热力学稳定性。此外,相对于TA,由于动力学和热力学劣势,可以排除产生副产物无环碳酸酯的可能性。该结果不同于有机碱介导的反应。或者,我们的计算预测,与TA的铯盐一起生成的CsHCO 3可能也是将CO 2与炔丙醇结合的一种可用的介导试剂。与其他碱金属碳酸盐M 2 CO 3相比(M = Li,Na,K),Cs 2 CO 3的强碱性和铯离子的较低离子电势可以提高αACC中间体的有效浓度,从而将αACC中间体转化为铯的铯盐。可以高收率获得TA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号