当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold Catalyzed Cyclopropanation/[5+3] Cycloaddition of 1,4,9‐ and 1,4,10‐Allenenynes to Bicyclo[3.3.1]nonane Derivatives
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901263 Xianxiao Chen 1 , Yuanyuan Zhou 1 , Jianwen Jin 2 , Kaveh Farshadfar 3 , Alireza Ariafard 3, 4 , Weidong Rao 1 , Philip Wai Hong Chan 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901263 Xianxiao Chen 1 , Yuanyuan Zhou 1 , Jianwen Jin 2 , Kaveh Farshadfar 3 , Alireza Ariafard 3, 4 , Weidong Rao 1 , Philip Wai Hong Chan 2
Affiliation
|
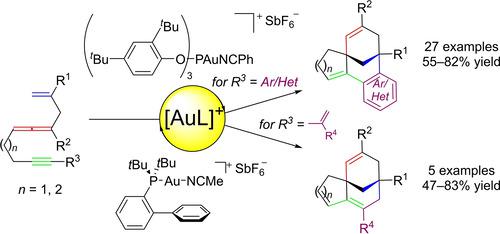
A synthetic method to prepare cycloalkyl‐ and (hetero)aryl‐fused bicyclo[3.3.1]nonane derivatives from gold(I)‐catalyzed cyclopropanation/[5+3] cycloaddition of 1,4,9‐ and 1,4,10‐allenenynes is described. The suggested double cycloisomerization mechanism delineates the first example of a stepwise [5+3] cycloaddition pathway in gold catalysis, a mode of reactivity that is also sparse in organic chemistry. Experimental and Density Functional Theory (DFT) computational studies based on a proposed gold carbenoid species provides insight into this unique selectivity that leads to the assembly of the architecturally challenging bridged carbocyclic motif.
中文翻译:

金催化的1,4,9-和1,4,10-烯丙炔的环丙烷化/ [5 + 3]环化成双环[3.3.1]壬烷衍生物
一种合成方法,由金(I)催化的1,4,9-和1,4,10的环丙烷化/ [5 + 3]环加成反应制备环烷基和(杂)芳基稠合的双环[3.3.1]壬烷衍生物描述了烯丙炔。所提出的双环异构化机理描述了金催化中逐步[5 + 3]环加成途径的第一个例子,该反应在有机化学中也是稀疏的。基于拟议的金类类胡萝卜素的实验和密度泛函理论(DFT)计算研究提供了对这种独特选择性的洞察力,该选择性导致了具有建筑挑战性的桥连碳环基序的组装。
更新日期:2020-01-17
中文翻译:

金催化的1,4,9-和1,4,10-烯丙炔的环丙烷化/ [5 + 3]环化成双环[3.3.1]壬烷衍生物
一种合成方法,由金(I)催化的1,4,9-和1,4,10的环丙烷化/ [5 + 3]环加成反应制备环烷基和(杂)芳基稠合的双环[3.3.1]壬烷衍生物描述了烯丙炔。所提出的双环异构化机理描述了金催化中逐步[5 + 3]环加成途径的第一个例子,该反应在有机化学中也是稀疏的。基于拟议的金类类胡萝卜素的实验和密度泛函理论(DFT)计算研究提供了对这种独特选择性的洞察力,该选择性导致了具有建筑挑战性的桥连碳环基序的组装。



























 京公网安备 11010802027423号
京公网安备 11010802027423号