当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study of 1,4-Benzodiazepine-2,5-diones from Diphenylamine and Diethyl 2-Phenylmalonate by Density Functional Theory.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpca.9b10662 Da-Gang Zhou 1 , Yan-Qiong Li 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpca.9b10662 Da-Gang Zhou 1 , Yan-Qiong Li 2
Affiliation
|
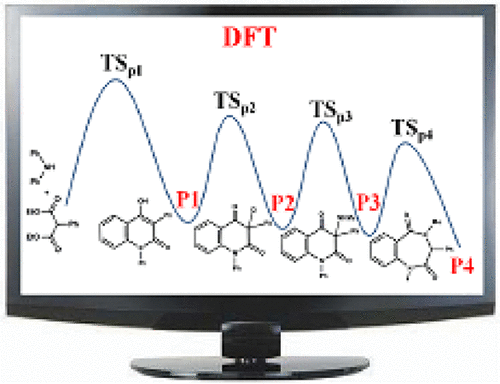
The mechanisms for the reaction between diphenylamine and diethyl 2-phenylmalonate were investigated using M06-2X-D3/6-31+G(d,p) method and level, and the SMD model was applied to simulate the solvent effect. The computational results suggested that diphenylamine and diethyl 2-phenylmalonate can convert into 4-hydroxy-1,3-diphenylquinolin-2(1H)-one via a series of reactions (addition reaction, dealcoholization reaction, enolization reaction, dealcoholization reaction, ring-closure reaction, and H-shift reaction). And H2O, as the catalyst, can play an important role to promote these reactions. In the following reaction, there are two paths to yield the second product 3-chloro-1,3-diphenylquinoline-2,4(1H,3H)-dione and the computational results indicated that the first path (blue line) with the rate-determining step of 24.9 kcal/mol is favorable. With the participation of methanamine, a SN2 reaction happened and the third product 3-(methylamino)-1,3-diphenylquinoline-2,4(1H,3H)-dione had been yielded in the effect of methanamine or Cl anion. The analysis of Gibbs free energy surfaces shows that methanamine is better than Cl anion to extract the proton via an exothermic reaction. Finally, the third product 3-(methylamino)-1,3-diphenylquinoline-2,4(1H,3H)-dione would go through a ring-enlargement reaction, promoted by base (TMG or Triton B), to yield the final product. The computational results demonstrated that this reaction can release much energy with Triton B than that with TMG. And the energy of the highest point is 10.1 kcal/mol (16.8 kcal/mol), which can readily occur at the room temperature. The results could provide valuable insights into these types of interactions and related ones.
中文翻译:

通过密度泛函理论研究二苯胺和2-苯基丙二酸二乙酯中的1,4-苯并二氮杂-2,5-二酮的机理。
用M06-2X-D3 / 6-31 + G(d,p)法和水平研究了二苯胺与2-苯基丙二酸二乙酯之间的反应机理,并采用SMD模型模拟了溶剂作用。计算结果表明二苯胺和2-苯基丙二酸二乙酯可通过一系列反应(加成反应,脱醇反应,烯醇化反应,脱醇反应,环-封闭反应和H移反应)。而H2O作为催化剂,可以在促进这些反应中发挥重要作用。在下面的反应中,有两种途径产生第二种产物3-氯-1,3-二苯基喹啉-2,4(1H,3H)-二酮,计算结果表明,第一途径(蓝线)与-24.9kcal / mol的确定步骤是有利的。在甲胺的参与下,发生了SN2反应,在甲胺或Cl阴离子的作用下,产生了第三种产物3-(甲基氨基)-1,3-二苯基喹啉-2,4(1H,3H)-二酮。吉布斯自由能表面的分析表明,甲胺通过放热反应比氯阴离子更好地萃取质子。最后,第三种产物3-(甲基氨基)-1,3-二苯基喹啉-2,4(1H,3H)-二酮将经历扩环反应,由碱(TMG或Triton B)促进,得到最终产物。产品。计算结果表明,与TMG相比,Triton B可以释放更多的能量。最高能量为10.1 kcal / mol(16.8 kcal / mol),在室温下很容易发生。
更新日期:2020-01-07
中文翻译:

通过密度泛函理论研究二苯胺和2-苯基丙二酸二乙酯中的1,4-苯并二氮杂-2,5-二酮的机理。
用M06-2X-D3 / 6-31 + G(d,p)法和水平研究了二苯胺与2-苯基丙二酸二乙酯之间的反应机理,并采用SMD模型模拟了溶剂作用。计算结果表明二苯胺和2-苯基丙二酸二乙酯可通过一系列反应(加成反应,脱醇反应,烯醇化反应,脱醇反应,环-封闭反应和H移反应)。而H2O作为催化剂,可以在促进这些反应中发挥重要作用。在下面的反应中,有两种途径产生第二种产物3-氯-1,3-二苯基喹啉-2,4(1H,3H)-二酮,计算结果表明,第一途径(蓝线)与-24.9kcal / mol的确定步骤是有利的。在甲胺的参与下,发生了SN2反应,在甲胺或Cl阴离子的作用下,产生了第三种产物3-(甲基氨基)-1,3-二苯基喹啉-2,4(1H,3H)-二酮。吉布斯自由能表面的分析表明,甲胺通过放热反应比氯阴离子更好地萃取质子。最后,第三种产物3-(甲基氨基)-1,3-二苯基喹啉-2,4(1H,3H)-二酮将经历扩环反应,由碱(TMG或Triton B)促进,得到最终产物。产品。计算结果表明,与TMG相比,Triton B可以释放更多的能量。最高能量为10.1 kcal / mol(16.8 kcal / mol),在室温下很容易发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号