当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acscatal.9b04699 Bin Zhao 1 , Rui Shang 1, 2 , Guang-Zu Wang 1 , Shaohong Wang 3 , Hui Chen 3 , Yao Fu 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acscatal.9b04699 Bin Zhao 1 , Rui Shang 1, 2 , Guang-Zu Wang 1 , Shaohong Wang 3 , Hui Chen 3 , Yao Fu 1
Affiliation
|
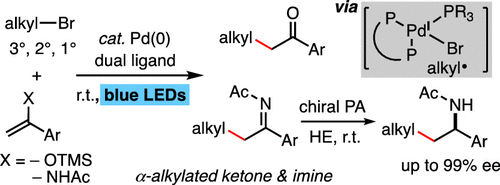
We report herein that a palladium catalyst in combination with a dual phosphine ligand system catalyzes alkylation of silyl enol ether and enamide with a broad scope of tertiary, secondary, and primary alkyl bromides under mild irradiation conditions by blue light-emitting diodes. The reactions effectively deliver α-alkylated ketones and α-alkylated N-acyl ketimines, and it is difficult to prepare the latter by other methods in a stereoselective manner. The α-alkylated N-acyl ketimine products can be further subjected to chiral phosphoric acid-catalyzed asymmetric reduction with Hantzsch ester to deliver chiral N-acyl-protected α-arylated aliphatic amines in high enantioselectivity up to 99% ee, thus providing a method for facile synthesis of chiral α-arylated aliphatic amines, which are of importance in medicinal chemistry research. The N-acetyl ketimine product also reacted smoothly with various types of Grignard reagents to afford sterically bulky N-acetyl α-tertiary amines in high yields. Theoretical studies in combination with experimental investigation provide understanding of the reaction mechanism with respect to the dual ligand effect and the irradiation effect in the catalytic cycle. The reaction is suggested to proceed via a hybrid alkyl Pd(I)-radical species generated by inner-sphere electron transfer of phosphine-coordinated Pd(0) species with alkyl bromide. This intriguing hybrid alkyl Pd(I)-radical species is elucidated by theoretical calculation to be a triplet species coordinated by three phosphine atoms with a distorted tetrahedral geometry, and spin prohibition rather than metal-to-ligand charge transfer contributes to the kinetic stability of the hybrid alkyl Pd(I)-radical species to impede alkyl recombination to generate Pd(II) alkyl intermediate.
中文翻译:

辐照下钯催化的硅烯醇醚和酰胺的双配体活化烷基化:杂烷基Pd(I)-自由基物种的范围,机理和理论解释
我们在此报道,钯催化剂与双膦配体系统结合在蓝色发光二极管的温和辐照条件下催化具有广泛范围的叔,仲和伯烷基溴的甲硅烷基烯醇醚和烯酰胺的烷基化。该反应有效地递送α-烷基化的酮和α-烷基化的N-酰基酮亚胺,并且难以通过其他方法以立体选择性的方式制备后者。α-烷基化的N-酰基酮亚胺产物可以进一步用Hantzsch酯进行手性磷酸催化的不对称还原,以递送手性N-酰基保护的α-芳基化脂肪族胺具有高达99%ee的高对映体选择性,因此提供了一种简便合成手性α-芳基化脂肪族胺的方法,这在药物化学研究中具有重要意义。的Ñ -乙酰基酮亚胺产物也与各种类型的格氏试剂的顺利反应,得到空间庞大Ñ-乙酰基α-叔胺的收率高。理论研究与实验研究相结合,使人们对催化循环中双配体效应和辐射效应的反应机理有了了解。建议该反应通过由膦配位的Pd(0)物质与烷基溴的内球电子转移产生的杂化烷基Pd(I)-自由基物质进行。通过理论计算,这种有趣的杂化烷基Pd(I)自由基基团是由三个具有四面体几何形状扭曲的膦原子配位的三重态物种,并且自旋抑制而不是金属到配体的电荷转移有助于分子的动力学稳定性。杂烷基Pd(I)自由基可阻止烷基重组以生成Pd(II)烷基中间体。
更新日期:2020-01-02
中文翻译:

辐照下钯催化的硅烯醇醚和酰胺的双配体活化烷基化:杂烷基Pd(I)-自由基物种的范围,机理和理论解释
我们在此报道,钯催化剂与双膦配体系统结合在蓝色发光二极管的温和辐照条件下催化具有广泛范围的叔,仲和伯烷基溴的甲硅烷基烯醇醚和烯酰胺的烷基化。该反应有效地递送α-烷基化的酮和α-烷基化的N-酰基酮亚胺,并且难以通过其他方法以立体选择性的方式制备后者。α-烷基化的N-酰基酮亚胺产物可以进一步用Hantzsch酯进行手性磷酸催化的不对称还原,以递送手性N-酰基保护的α-芳基化脂肪族胺具有高达99%ee的高对映体选择性,因此提供了一种简便合成手性α-芳基化脂肪族胺的方法,这在药物化学研究中具有重要意义。的Ñ -乙酰基酮亚胺产物也与各种类型的格氏试剂的顺利反应,得到空间庞大Ñ-乙酰基α-叔胺的收率高。理论研究与实验研究相结合,使人们对催化循环中双配体效应和辐射效应的反应机理有了了解。建议该反应通过由膦配位的Pd(0)物质与烷基溴的内球电子转移产生的杂化烷基Pd(I)-自由基物质进行。通过理论计算,这种有趣的杂化烷基Pd(I)自由基基团是由三个具有四面体几何形状扭曲的膦原子配位的三重态物种,并且自旋抑制而不是金属到配体的电荷转移有助于分子的动力学稳定性。杂烷基Pd(I)自由基可阻止烷基重组以生成Pd(II)烷基中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号