当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct synthesis of 2-arylazulenes by [8+2] cycloaddition of 2H-cyclohepta[b]furan-2-ones with silyl enol ethers.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cc09376a Taku Shoji 1 , Shuhei Sugiyama , Yoshiaki Kobayashi , Akari Yamazaki , Yukino Ariga , Ryuzi Katoh , Hiroki Wakui , Masafumi Yasunami , Shunji Ito
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cc09376a Taku Shoji 1 , Shuhei Sugiyama , Yoshiaki Kobayashi , Akari Yamazaki , Yukino Ariga , Ryuzi Katoh , Hiroki Wakui , Masafumi Yasunami , Shunji Ito
Affiliation
|
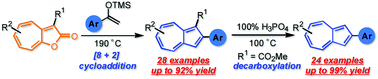
We developed a procedure for the direct synthesis of 2-arylazulenes, which were obtained in moderate to excellent yields, by [8+2] cycloaddition of 2H-cyclohepta[b]furan-2-ones with aryl-substituted silyl enol ethers. The structures of some 2-arylazulenes were clarified by single-crystal X-ray analysis. The 2-phenylazulene derivatives obtained by this study showed noticeable fluorescence in acidic media.
中文翻译:

通过将2H-环庚基[b]呋喃-2-酮与甲硅烷基烯醇醚进行[8 + 2]环加成反应,可以直接合成2-芳基az烯。
我们开发了一种直接合成2-芳基azulenes的程序,该方法可以通过2H-环庚[b]呋喃-2-酮与芳基取代的甲硅烷基烯醇醚的[8 + 2]环加成反应而以中等至极好的收率获得。通过单晶X射线分析澄清了一些2-芳基az嗪的结构。通过该研究获得的2-苯基氮杂烯衍生物在酸性介质中显示出明显的荧光。
更新日期:2020-01-08
中文翻译:

通过将2H-环庚基[b]呋喃-2-酮与甲硅烷基烯醇醚进行[8 + 2]环加成反应,可以直接合成2-芳基az烯。
我们开发了一种直接合成2-芳基azulenes的程序,该方法可以通过2H-环庚[b]呋喃-2-酮与芳基取代的甲硅烷基烯醇醚的[8 + 2]环加成反应而以中等至极好的收率获得。通过单晶X射线分析澄清了一些2-芳基az嗪的结构。通过该研究获得的2-苯基氮杂烯衍生物在酸性介质中显示出明显的荧光。











































 京公网安备 11010802027423号
京公网安备 11010802027423号