当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Usnic Acid Enaminone-Coupled 1,2,3-Triazoles as Antibacterial and Antitubercular Agents.
Journal of Natural Products ( IF 5.1 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jnatprod.9b00475 Pavan K Bangalore , Siva K Vagolu 1 , Rakesh K Bollikanda , Dileep K Veeragoni , Pallavi C Choudante , Sunil Misra , Dharmarajan Sriram 1 , Balasubramanian Sridhar , Srinivas Kantevari
Journal of Natural Products ( IF 5.1 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jnatprod.9b00475 Pavan K Bangalore , Siva K Vagolu 1 , Rakesh K Bollikanda , Dileep K Veeragoni , Pallavi C Choudante , Sunil Misra , Dharmarajan Sriram 1 , Balasubramanian Sridhar , Srinivas Kantevari
Affiliation
|
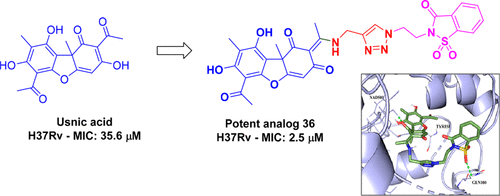
(+)-Usnic acid, a product of secondary metabolism in lichens, has displayed a broad range of biological properties such as antitumor, antimicrobial, antiviral, anti-inflammatory, and insecticidal activities. Interested by these pharmacological activities and to tap into its potential, we herein present the synthesis and biological evaluation of new usnic acid enaminone-conjugated 1,2,3-triazoles 10-44 as antimycobacterial agents. (+)-Usnic acid was condensed with propargyl amine to give usnic acid enaminone 8 with a terminal ethynyl moiety. It was further reacted with various azides A1-A35 under copper catalysis to give triazoles 10-44 in good yields. Among the synthesized compounds, saccharin derivative 36 proved to be the most active analogue, inhibiting Mycobacterium tuberculosis (Mtb) at an MIC value of 2.5 μM. Analogues 16 and 27, with 3,4-difluorophenacyl and 2-acylnaphthalene units, respectively, inhibited Mtb at MIC values of 5.4 and 5.3 μM, respectively. Among the tested Gram-positive and Gram-negative bacteria, the new derivatives were active on Bacillus subtilis, with compounds 18 [3-(trifluoromethyl)phenacyl] and 29 (N-acylmorpholinyl) showing inhibitory concentrations of 41 and 90.7 μM, respectively, while they were inactive on the other tested bacterial strains. Overall, the study presented here is useful for converting natural (+)-usnic acid into antitubercular and antibacterial agents via incorporation of enaminone and 1,2,3-triazole functionalities.
中文翻译:

松萝酸烯胺酮偶联的1,2,3-三唑类用作抗菌剂和抗结核剂。
(+)-尿酸是地衣中的二次代谢产物,具有广泛的生物学特性,例如抗肿瘤,抗微生物,抗病毒,抗炎和杀虫活性。对这些药理活性感兴趣并挖掘其潜力,我们在此介绍新的松萝酸烯胺酮偶联的1,2,3-三唑10-44作为抗分枝杆菌药的合成和生物学评估。将(+)-松香酸与炔丙基胺缩合,得到具有末端乙炔基部分的松香酸烯胺酮8。在铜催化下,它进一步与各种叠氮化物A1-A35反应,以高收率得到三唑10-44。在合成的化合物中,糖精衍生物36被证明是活性最高的类似物,在MIC值为2.5μM时可抑制结核分枝杆菌(Mtb)。类似物16和27,以及3,4-二氟苯酰基和2-酰基萘单元分别在MIC值为5.4和5.3μM时抑制Mtb。在测试的革兰氏阳性和革兰氏阴性细菌中,新衍生物对枯草芽孢杆菌具有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而它们对其他测试细菌菌株没有活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。新的衍生物对枯草芽孢杆菌有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而对其他测试的细菌菌株则无活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。新衍生物对枯草芽孢杆菌具有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而对其他测试细菌菌株则无活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。
更新日期:2019-12-20
中文翻译:

松萝酸烯胺酮偶联的1,2,3-三唑类用作抗菌剂和抗结核剂。
(+)-尿酸是地衣中的二次代谢产物,具有广泛的生物学特性,例如抗肿瘤,抗微生物,抗病毒,抗炎和杀虫活性。对这些药理活性感兴趣并挖掘其潜力,我们在此介绍新的松萝酸烯胺酮偶联的1,2,3-三唑10-44作为抗分枝杆菌药的合成和生物学评估。将(+)-松香酸与炔丙基胺缩合,得到具有末端乙炔基部分的松香酸烯胺酮8。在铜催化下,它进一步与各种叠氮化物A1-A35反应,以高收率得到三唑10-44。在合成的化合物中,糖精衍生物36被证明是活性最高的类似物,在MIC值为2.5μM时可抑制结核分枝杆菌(Mtb)。类似物16和27,以及3,4-二氟苯酰基和2-酰基萘单元分别在MIC值为5.4和5.3μM时抑制Mtb。在测试的革兰氏阳性和革兰氏阴性细菌中,新衍生物对枯草芽孢杆菌具有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而它们对其他测试细菌菌株没有活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。新的衍生物对枯草芽孢杆菌有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而对其他测试的细菌菌株则无活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。新衍生物对枯草芽孢杆菌具有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而对其他测试细菌菌株则无活性。总体而言,此处介绍的研究可通过结合烯胺酮和1,2,3-三唑官能团将天然(+)-松香酸转化为抗结核药和抗菌剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号