当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of Orally Bioavailable PI3Kδ Inhibitors and Identification of Vps34 as a Key Selectivity Target.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jmedchem.9b01585 Zoë A Henley , Augustin Amour , Nick Barton , Marcus Bantscheff 1 , Giovanna Bergamini 1 , Sophie M Bertrand , Máire Convery , Kenneth Down , Birgit Dümpelfeld 1 , Chris D Edwards , Paola Grandi 1 , Paul M Gore , Steve Keeling , Stefano Livia , David Mallett , Aoife Maxwell , Mark Price 2 , Christina Rau 1 , Friedrich B M Reinhard 1 , James Rowedder , Paul Rowland , Jonathan A Taylor , Daniel A Thomas , Edith M Hessel , J Nicole Hamblin
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jmedchem.9b01585 Zoë A Henley , Augustin Amour , Nick Barton , Marcus Bantscheff 1 , Giovanna Bergamini 1 , Sophie M Bertrand , Máire Convery , Kenneth Down , Birgit Dümpelfeld 1 , Chris D Edwards , Paola Grandi 1 , Paul M Gore , Steve Keeling , Stefano Livia , David Mallett , Aoife Maxwell , Mark Price 2 , Christina Rau 1 , Friedrich B M Reinhard 1 , James Rowedder , Paul Rowland , Jonathan A Taylor , Daniel A Thomas , Edith M Hessel , J Nicole Hamblin
Affiliation
|
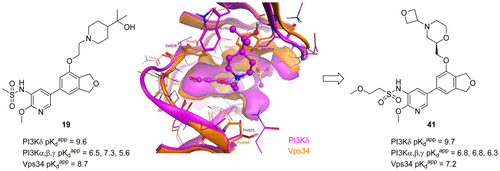
Optimization of a lead series of PI3Kδ inhibitors based on a dihydroisobenzofuran core led to the identification of potent, orally bioavailable compound 19. Selectivity profiling of compound 19 showed similar potency for class III PI3K, Vps34, and PI3Kδ, and compound 19 was not well-tolerated in a 7-day rat toxicity study. Structure-based design led to an improvement in selectivity for PI3Kδ over Vps34 and, a focus on oral phramacokinetics properties resulted in the discovery of compound 41, which showed improved toxicological outcomes at similar exposure levels to compound 19.
中文翻译:

口服生物利用PI3Kδ抑制剂的优化和作为主要选择性目标的Vps34的鉴定。
基于二氢异苯并呋喃核的PI3Kδ抑制剂先导系列的优化导致鉴定了有效的,口服生物利用度高的化合物19。化合物19的选择性分析显示了对III类PI3K,Vps34和PI3Kδ的相似效价,而化合物19的效果不佳-在7天的大鼠毒性研究中耐受。基于结构的设计导致PI3Kδ的选择性相对于Vps34有所改善,并且对口服言语动力学特性的关注导致发现了化合物41,该化合物在与化合物19相似的暴露水平下显示出改善的毒理学结果。
更新日期:2020-01-08
中文翻译:

口服生物利用PI3Kδ抑制剂的优化和作为主要选择性目标的Vps34的鉴定。
基于二氢异苯并呋喃核的PI3Kδ抑制剂先导系列的优化导致鉴定了有效的,口服生物利用度高的化合物19。化合物19的选择性分析显示了对III类PI3K,Vps34和PI3Kδ的相似效价,而化合物19的效果不佳-在7天的大鼠毒性研究中耐受。基于结构的设计导致PI3Kδ的选择性相对于Vps34有所改善,并且对口服言语动力学特性的关注导致发现了化合物41,该化合物在与化合物19相似的暴露水平下显示出改善的毒理学结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号