当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,3,4-trisubstituted pyrrole derivatives via [3 + 2] cyclization of activated methylene isocyanides with 4-(arylidene)-2-substituted oxazol-5(4H)-ones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019/12/19 , DOI: 10.1039/c9qo01044k Jing Zhang 1, 2, 3, 4, 5 , Man Liu 4, 5, 6, 7, 8 , Chao Li 4, 5, 6, 7, 8 , Yan-Jun Xu 1, 2, 3, 4 , Lin Dong 4, 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019/12/19 , DOI: 10.1039/c9qo01044k Jing Zhang 1, 2, 3, 4, 5 , Man Liu 4, 5, 6, 7, 8 , Chao Li 4, 5, 6, 7, 8 , Yan-Jun Xu 1, 2, 3, 4 , Lin Dong 4, 5, 6, 7, 8
Affiliation
|
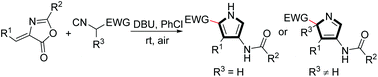
An efficient base-catalyzed [3 + 2] cyclization between isocyanides and 4-(arylidene)-2-substituted oxazol-5(4H)-ones has been successfully developed to form 2,3,4-trisubstituted and 2,2,3,4-tetrasubstituted pyrrole derivatives. This transition metal free reaction occurs at room temperature under environmentally friendly conditions, tolerating water and air.
中文翻译:

通过[3 + 2]与4-(亚芳基)-2-取代的恶唑-5(4H)-的活化亚甲基异氰酸酯的环化反应合成2,3,4-三取代的吡咯衍生物
已成功开发出异氰酸酯与4-(亚芳基)-2-取代的恶唑-5(4 H)-酮之间有效的碱催化[3 + 2]环化反应,形成2,3,4-三取代和2,2, 3,4-四取代的吡咯衍生物。这种无过渡金属的反应在室温,环境友好的条件下发生,可以耐受水和空气。
更新日期:2020-02-13
中文翻译:

通过[3 + 2]与4-(亚芳基)-2-取代的恶唑-5(4H)-的活化亚甲基异氰酸酯的环化反应合成2,3,4-三取代的吡咯衍生物
已成功开发出异氰酸酯与4-(亚芳基)-2-取代的恶唑-5(4 H)-酮之间有效的碱催化[3 + 2]环化反应,形成2,3,4-三取代和2,2, 3,4-四取代的吡咯衍生物。这种无过渡金属的反应在室温,环境友好的条件下发生,可以耐受水和空气。











































 京公网安备 11010802027423号
京公网安备 11010802027423号