当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine(iii) promoted ring-rearrangement reaction of 1-arylamino-2-oxocyclopentane-1-carbonitriles to synthesize N-aryl-δ-valerolactams.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9ob02598g Dhananjay Bhattacherjee 1 , Shaifali 2 , Ajay Kumar 2 , Ajay Sharma 3 , Rituraj Purohit 4 , Pralay Das 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9ob02598g Dhananjay Bhattacherjee 1 , Shaifali 2 , Ajay Kumar 2 , Ajay Sharma 3 , Rituraj Purohit 4 , Pralay Das 2
Affiliation
|
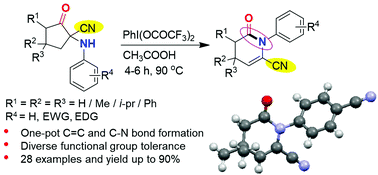
The first example of N-aryl-δ-valerolactam synthesis via an intramolecular ring rearrangement reaction of 1-arylamino-2-oxocyclopentane-1-carbonitrile promoted by phenyliodine bis(trifluoroacetate) (PIFA) was reported. We show that this unprecedented regio-selective ring-rearrangement reaction driven by hypervalent iodine (PIFA) involves C5-H elimination, C1-C2 bond opening, and C1-N bond rearrangement steps and restraining the leaving tendency of the CN group. The structure of the lactam was further confirmed by single crystal X-ray diffraction (XRD) analysis. The present protocol showed a diverse array of functional group tolerance under the reaction conditions and offered good to excellent yields of lactams.
中文翻译:

碘(iii)促进了1-芳基氨基-2-氧代环戊烷-1-腈的环重排反应,从而合成了N-芳基-δ-戊内酰胺。
报道了通过苯基碘双(三氟乙酸)酯(PIFA)促进的1-芳基氨基-2-氧代环戊烷-1-腈的分子内环重排反应合成N-芳基-δ-戊内酰胺的第一个实例。我们显示,这种由高价碘(PIFA)驱动的前所未有的区域选择性环重排反应涉及C5-H消除,C1-C2键打开和C1-N键重排步骤以及抑制CN基团的离去趋势。内酰胺的结构通过单晶X射线衍射(XRD)分析进一步证实。本方案在反应条件下显示出各种不同的官能团耐受性,并提供了良好的内酰胺收率。
更新日期:2020-02-10
中文翻译:

碘(iii)促进了1-芳基氨基-2-氧代环戊烷-1-腈的环重排反应,从而合成了N-芳基-δ-戊内酰胺。
报道了通过苯基碘双(三氟乙酸)酯(PIFA)促进的1-芳基氨基-2-氧代环戊烷-1-腈的分子内环重排反应合成N-芳基-δ-戊内酰胺的第一个实例。我们显示,这种由高价碘(PIFA)驱动的前所未有的区域选择性环重排反应涉及C5-H消除,C1-C2键打开和C1-N键重排步骤以及抑制CN基团的离去趋势。内酰胺的结构通过单晶X射线衍射(XRD)分析进一步证实。本方案在反应条件下显示出各种不同的官能团耐受性,并提供了良好的内酰胺收率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号