当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the heme protein hemoglobin by (-)-epigallocatechin gallate and the study of polyphenol-protein association using multi-spectroscopic and computational methods.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp05301h Sourav Das 1 , Sharat Sarmah , Zaved Hazarika , Mostofa Ataur Rohman , Pallavi Sarkhel , Anupam Nath Jha , Atanu Singha Roy
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp05301h Sourav Das 1 , Sharat Sarmah , Zaved Hazarika , Mostofa Ataur Rohman , Pallavi Sarkhel , Anupam Nath Jha , Atanu Singha Roy
Affiliation
|
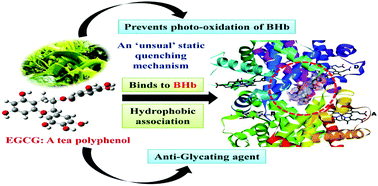
In this work, the interaction of a bioactive tea polyphenol (-)-epigallocatechin gallate (EGCG) with bovine hemoglobin (BHb) along with its anti-oxidative behavior and the anti-glycation property have been explored using multi-spectroscopic and computational techniques. The binding affinity for EGCG towards BHb was observed to be moderate in nature with an order of 104 M-1, and the fluorescence quenching mechanism was characterized by an unusual static quenching mechanism. The binding constant (Kb) showed a continuous enhancement with temperature from 3.468 ± 0.380 × 104 M-1 at 288 K to 6.017 ± 0.601 × 104 M-1 at 310 K. The fluorescence emission measurements along with molecular docking studies indicated that EGCG binds near the most dominant fluorophore of BHb (β2-Trp37, at the interface of α1 and β2 chains) within the pocket formed by the α1, α2 and β2 chains. The sign and magnitude of the thermodynamic parameters, changes in enthalpy (ΔH = +17.004 ± 1.007 kJ mol-1) and in entropy (ΔS = +146.213 ± 2.390 J K-1 mol-1), indicate that hydrophobic forces play a major role in stabilizing the BHb-EGCG complex. The micro-environment around the EGCG binding site showed an increase in hydrophobicity upon ligand binding. The binding of EGCG with BHb leads to a decrease in the α-helical content, whereas that of the β-sheet increased. FTIR studies also indicated that the secondary structure of BHb changed upon binding with EGCG, along with providing further support for the presence of hydrophobic forces in the complexation process. Molecular docking studies indicated that EGCG binds within the cavity of α1, α2, and β2 chains surrounded by residues such as α1- Lys99, α1-Thr134, α1-Thr137, α1-Tyr140, α2-Lys127 and β2-Trp37. Molecular dynamics simulation studies indicated that EGCG conferred additional stability to BHb. Furthermore, moving away from the binding studies, EGCG was found to prevent the glyoxal (GO)-mediated glycation process of BHb, and it was also found to act as a potent antioxidant against the photo-oxidative damage of BHb.
中文翻译:

通过(-)-表没食子儿茶素没食子酸酯靶向血红素蛋白血红蛋白,并使用多光谱和计算方法研究多酚-蛋白缔合。
在这项工作中,已使用多光谱和计算技术探索了具有生物活性的茶多酚(-)-表没食子儿茶素没食子酸酯(EGCG)与牛血红蛋白(BHb)的相互作用以及其抗氧化行为和抗糖基化特性。观察到对EGCG对BHb的结合亲和力本质上是中等的,为104 M-1,并且荧光猝灭机制的特征在于异常的静态猝灭机制。结合常数(Kb)随温度从288 K时的3.468±0.380×104 M-1到310 K时的6.017±0.601×104 M-1连续增强。荧光发射测量和分子对接研究表明EGCG结合在由α1形成的囊袋中,靠近BHb最主要的荧光团(β2-Trp37,在α1和β2链的界面处),α2和β2链。热力学参数的符号和大小,焓(ΔH= +17.004±1.007 kJ mol-1)和熵(ΔS= +146.213±2.390 J K-1 mol-1)的变化表明,疏水力起主要作用在稳定BHb-EGCG复合物中的作用。EGCG结合位点周围的微环境显示出配体结合后疏水性的增加。EGCG与BHb的结合导致α-螺旋含量的减少,而β-折叠的含量增加。FTIR研究还表明,BHb的二级结构在与EGCG结合后发生了变化,并为络合过程中疏水力的存在提供了进一步的支持。分子对接研究表明,EGCG结合在被残基如α1-Lys99,α1-Thr134,α1-Thr137,α1-Tyr140,α2-Lys127和β2-Trp37。分子动力学模拟研究表明,EGCG赋予BHb额外的稳定性。此外,远离结合研究,发现EGCG可以防止乙二醛(GO)介导的BHb糖基化过程,并且它还可以作为有效的抗氧化剂来抵抗BHb的光氧化损伤。
更新日期:2020-01-08
中文翻译:

通过(-)-表没食子儿茶素没食子酸酯靶向血红素蛋白血红蛋白,并使用多光谱和计算方法研究多酚-蛋白缔合。
在这项工作中,已使用多光谱和计算技术探索了具有生物活性的茶多酚(-)-表没食子儿茶素没食子酸酯(EGCG)与牛血红蛋白(BHb)的相互作用以及其抗氧化行为和抗糖基化特性。观察到对EGCG对BHb的结合亲和力本质上是中等的,为104 M-1,并且荧光猝灭机制的特征在于异常的静态猝灭机制。结合常数(Kb)随温度从288 K时的3.468±0.380×104 M-1到310 K时的6.017±0.601×104 M-1连续增强。荧光发射测量和分子对接研究表明EGCG结合在由α1形成的囊袋中,靠近BHb最主要的荧光团(β2-Trp37,在α1和β2链的界面处),α2和β2链。热力学参数的符号和大小,焓(ΔH= +17.004±1.007 kJ mol-1)和熵(ΔS= +146.213±2.390 J K-1 mol-1)的变化表明,疏水力起主要作用在稳定BHb-EGCG复合物中的作用。EGCG结合位点周围的微环境显示出配体结合后疏水性的增加。EGCG与BHb的结合导致α-螺旋含量的减少,而β-折叠的含量增加。FTIR研究还表明,BHb的二级结构在与EGCG结合后发生了变化,并为络合过程中疏水力的存在提供了进一步的支持。分子对接研究表明,EGCG结合在被残基如α1-Lys99,α1-Thr134,α1-Thr137,α1-Tyr140,α2-Lys127和β2-Trp37。分子动力学模拟研究表明,EGCG赋予BHb额外的稳定性。此外,远离结合研究,发现EGCG可以防止乙二醛(GO)介导的BHb糖基化过程,并且它还可以作为有效的抗氧化剂来抵抗BHb的光氧化损伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号