当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of Rhodium‐Catalyzed C−C Activation of Cyclobutanones: Origin of Ligand‐Controlled Product Selectivity
ChemCatChem ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1002/cctc.201902069 Tian Zhang 1 , Xiajun Wu 1 , Juan Li 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1002/cctc.201902069 Tian Zhang 1 , Xiajun Wu 1 , Juan Li 1
Affiliation
|
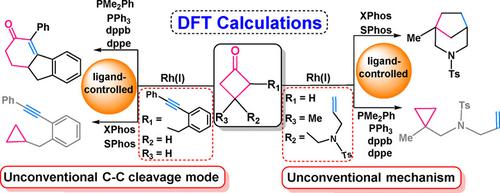
Cyclobutanone C−C activation with a Rh(I) catalyst has great potential for the synthesis of fused‐ and bridged‐ring systems. However, this synthetic application is greatly limited because of the direct CO extrusion from cyclobutanone, which leads to formation of cyclopropane as a byproduct. The calculations rationalize the experimental puzzles: why PMe2Ph and XPhos ligands can prevent cyclopropane formation for C2‐ and C3‐substituted cyclobutanone, respectively. More importantly, we enriched ligand computationally to exemplify how to develop new ligands. The small monodentate or bidentate phosphine ligand can favor [4+2] cycloaddition over cyclopropanation in the C2‐substituted cyclobutanone system. For the C3‐substituted cyclobutanone system, [4+2–1] cycloaddition is favored and cyclopropanation can be avoided when the large monodentate phosphine ligand is present. The different ligand requirements for C2‐ and C3‐substituted cyclobutanones are attributed to different mechanisms.
中文翻译:

铑催化的环丁酮CC活化的理论研究:配体控制产物选择性的起源
用Rh(I)催化剂活化环丁酮C-C在合成稠环和桥环系统中具有巨大潜力。但是,由于从环丁酮直接CO挤出,导致了副产物环丙烷的形成,因此这种合成应用受到极大限制。计算合理化了实验难题:为什么使用PMe 2Ph和XPhos配体可分别防止C2和C3取代的环丁酮形成环丙烷。更重要的是,我们在计算上丰富了配体,以举例说明如何开发新的配体。在C2取代的环丁酮系统中,小的单齿或双齿膦配体比环丙烷化更倾向于[4 + 2]环加成。对于C3取代的环丁酮体系,当存在较大的单齿膦配体时,[4 + 2-1]环加成是有利的,并且可以避免环丙烷化。C2和C3取代的环丁酮的不同配体要求归因于不同的机理。
更新日期:2020-01-17
中文翻译:

铑催化的环丁酮CC活化的理论研究:配体控制产物选择性的起源
用Rh(I)催化剂活化环丁酮C-C在合成稠环和桥环系统中具有巨大潜力。但是,由于从环丁酮直接CO挤出,导致了副产物环丙烷的形成,因此这种合成应用受到极大限制。计算合理化了实验难题:为什么使用PMe 2Ph和XPhos配体可分别防止C2和C3取代的环丁酮形成环丙烷。更重要的是,我们在计算上丰富了配体,以举例说明如何开发新的配体。在C2取代的环丁酮系统中,小的单齿或双齿膦配体比环丙烷化更倾向于[4 + 2]环加成。对于C3取代的环丁酮体系,当存在较大的单齿膦配体时,[4 + 2-1]环加成是有利的,并且可以避免环丙烷化。C2和C3取代的环丁酮的不同配体要求归因于不同的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号