当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Compensation Effect Mechanism of Fe–Ni Mixed Prussian Blue Analogues in Aqueous Rechargeable Aluminum‐Ion Batteries
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-27 , DOI: 10.1002/cssc.201903067 Yaning Gao 1 , Haoyi Yang 1 , Xinran Wang 1 , Ying Bai 1 , Na Zhu 1 , Shuainan Guo 1 , Liumin Suo 2 , Hong Li 2 , Huajie Xu 3 , Chuan Wu 1, 4
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-27 , DOI: 10.1002/cssc.201903067 Yaning Gao 1 , Haoyi Yang 1 , Xinran Wang 1 , Ying Bai 1 , Na Zhu 1 , Shuainan Guo 1 , Liumin Suo 2 , Hong Li 2 , Huajie Xu 3 , Chuan Wu 1, 4
Affiliation
|
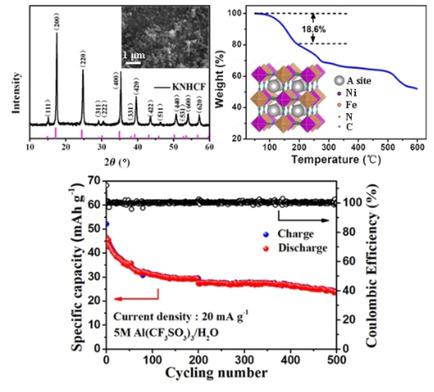
An aluminum‐ion battery was assembled with potassium nickel hexacyanoferrate (KNHCF) as a cathode and Al foil as an anode in aqueous electrolyte for the first time, based on Al3+ intercalation and deintercalation. A combination of ex situ XRD, X‐ray photoelectron spectroscopy (XPS), galvanostatic intermittent titration technique (GITT), and differential capacity analysis was used to unveil the crystal structure changes and the insertion/extraction mechanism of Al3+. Al3+ could reversibly insert/extract into/from KNHCF nanoparticles through a single‐phase reaction with reduction/oxidation of Fe and Ni. Over long‐term cycling, it was Fe rather than Ni that contributed to more capacity owing to the dissolution of Ni from the KNHCF structure, which could be expressed as a compensation effect of mixed redox centers in KNHCF. KNHCF delivered an initial discharge capacity of 46.5 mAh g−1. The capacity decay could be attributed to the unstable interface between Al foil and the aqueous electrolyte owing to the catalytic activity of the Ni transferring from Ni dissolution of KNHCF to the Al foil anode, rather than KNHCF structure collapse; KNHCF maintained its 3 D framework structure for 500 cycles. This work is expected to inspire more exhaustive investigations of the mechanisms that occur in aluminum‐ion batteries.
中文翻译:

Fe-Ni混合普鲁士蓝类似物在可充电铝离子电池中的补偿效应机理
基于Al 3+嵌入和脱嵌,首次将铝酸六氰合铁酸钾镍(KNHCF)用作阴极,将铝箔作为阳极组装在铝离子电池中。利用异位XRD,X射线光电子能谱(XPS),恒电流间歇滴定技术(GITT)和微分容量分析相结合,揭示了Al 3+的晶体结构变化和插入/提取机理。铝3+可以通过铁和镍的还原/氧化的单相反应可逆地从KNHCF纳米粒子中插入/提取。在长期循环中,由于镍从KNHCF结构中溶出,是铁而不是Ni贡献了更多的容量,这可以表示为KNHCF中混合氧化还原中心的补偿作用。KNHCF的初始放电容量为46.5 mAh g -1。容量下降可能归因于Al箔和水电解质之间的不稳定界面,这是由于Ni的催化活性从KNHCF的Ni溶解转移到Al箔阳极而引起的,而不是KNHCF的结构崩溃。KNHCF维持其3D框架结构500个循环。这项工作有望激发人们对铝离子电池中发生的机理进行更详尽的研究。
更新日期:2020-01-27
中文翻译:

Fe-Ni混合普鲁士蓝类似物在可充电铝离子电池中的补偿效应机理
基于Al 3+嵌入和脱嵌,首次将铝酸六氰合铁酸钾镍(KNHCF)用作阴极,将铝箔作为阳极组装在铝离子电池中。利用异位XRD,X射线光电子能谱(XPS),恒电流间歇滴定技术(GITT)和微分容量分析相结合,揭示了Al 3+的晶体结构变化和插入/提取机理。铝3+可以通过铁和镍的还原/氧化的单相反应可逆地从KNHCF纳米粒子中插入/提取。在长期循环中,由于镍从KNHCF结构中溶出,是铁而不是Ni贡献了更多的容量,这可以表示为KNHCF中混合氧化还原中心的补偿作用。KNHCF的初始放电容量为46.5 mAh g -1。容量下降可能归因于Al箔和水电解质之间的不稳定界面,这是由于Ni的催化活性从KNHCF的Ni溶解转移到Al箔阳极而引起的,而不是KNHCF的结构崩溃。KNHCF维持其3D框架结构500个循环。这项工作有望激发人们对铝离子电池中发生的机理进行更详尽的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号