Molecular Catalysis ( IF 3.9 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.mcat.2019.110741 Guochao Xu , Wei Dai , Yue Wang , Lu Zhang , Zewen Sun , Jieyu Zhou , Ye Ni
|
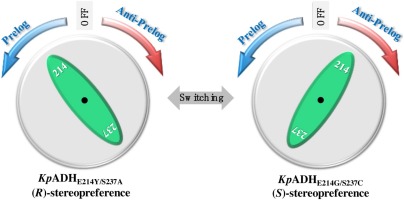
Structure-guided rational design revealed the molecular switch manipulating the Prelog and anti-Prelog priorities of an NADPH-dependent alcohol dehydrogenase toward prochiral ketones with bulky and similar substituents. Synergistic effects of unconserved residues at 214 and 237 in small and large substrate binding pockets were proven to be vital in governing the stereoselectivity. The ee values of E214Y/S237A and E214C/S237 G toward (4-chlorophenyl)-(pyridin-2-yl)-methanone were 99.3% (R) and 78.8% (S) respectively. Substrate specificity analysis revealed that similar patterns were also found with (4’-chlorophenyl)-phenylmethanone, (4’-bromophenyl)-phenylmethanone and (4’-nitrophenyl)-phenylmethanone. This study provides valuable evidence for understanding the molecular mechanism on enantioselective recognition of prochiral ketones by alcohol dehydrogenase.
中文翻译:

分子开关操纵醇脱氢酶对大体积酮的优先级
结构指导的合理设计揭示了分子开关操纵着NADPH依赖的醇脱氢酶的Prelog和anti-Prelog优先级向具有庞大和相似取代基的手性酮。事实证明,大小不一的底物结合袋中214和237处未保守残基的协同作用对于控制立体选择性至关重要。E214Y / S237A和E214C / S237 G对(4-氯苯基)-(吡啶-2-基)-甲酮的ee值分别为99.3%(R)和78.8%(S) 分别。底物特异性分析表明,(4'-氯苯基)-苯甲酮,(4'-溴苯基)-苯甲酮和(4'-硝基苯基)-苯甲酮也发现了相似的模式。该研究为理解醇脱氢酶对手性酮的对映选择性识别的分子机理提供了有价值的证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号