当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and validation of a Level A in-vitro in-vivo correlation for tofacitinib modified-release tablets using extrudable core system osmotic delivery technology.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.ejps.2019.105200 Joseph Kushner 1 , Manisha Lamba 1 , Thomas Stock 2 , Ronnie Wang 1 , Mary Anne Nemeth 1 , Christine Alvey 1 , Raymond Chen 1 , Vincent DeMatteo 1 , Andrew Blanchard 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.ejps.2019.105200 Joseph Kushner 1 , Manisha Lamba 1 , Thomas Stock 2 , Ronnie Wang 1 , Mary Anne Nemeth 1 , Christine Alvey 1 , Raymond Chen 1 , Vincent DeMatteo 1 , Andrew Blanchard 1
Affiliation
|
PURPOSE
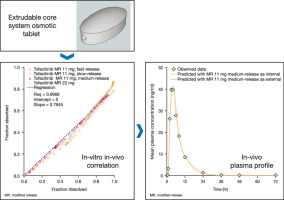
To determine if a validated Level A in-vitro in-vivo correlation (IVIVC) could be achieved with the extrudable core system (ECS) osmotic tablet platform. Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis.
METHODS
Fast-, medium-, and slow-release modified-release formulations of 11 mg tofacitinib ECS tablets, and one formulation of 22 mg tofacitinib ECS tablet, were manufactured. In vitro dissolution of the tofacitinib ECS tablets was performed using USP Apparatus 2 (paddles) and in vivo pharmacokinetic (PK) data were obtained from a Phase 1 study in healthy volunteers. A 5 mg immediate-release formulation tablet was included to support deconvolution of the tofacitinib ECS PK tablet data to obtain the in vivo absorption profiles. A linear, piecewise correlation and a simple linear correlation were used to build and validate two IVIVC models.
RESULTS
The prediction errors (PEs) for the linear, piecewise correlation met the Food and Drug Administration's criteria for establishing a Level A IVIVC, with a maximum absolute individual internal PE of 4.6%, a maximum absolute average internal PE of 3.9%, and a maximum absolute external PE of 8.4% obtained.
CONCLUSIONS
This study demonstrates that the tofacitinib ECS osmotic tablet platform can achieve a Level A IVIVC, similar to other osmotic delivery systems.
中文翻译:

使用可挤压核心系统渗透递送技术开发和验证托法替尼缓释片的A级体外相关性。
目的确定可挤出核心系统(ECS)渗透片剂平台是否可以实现经过验证的体外A级体内相关性(IVIVC)。Tofacitinib是用于治疗类风湿关节炎的口服JAK抑制剂。方法制备11 mg托法替尼ECS片剂的快速,中度和缓释缓释制剂,以及22 mg托法替尼ECS片剂的一种制剂。托法替尼ECS片剂的体外溶解是使用USP仪器2(桨)进行的,并且从健康志愿者的1期研究中获得了体内药代动力学(PK)数据。包含5 mg速释制剂片剂,以支持托法替尼ECS PK片剂数据解卷积以获得体内吸收曲线。线性的 使用分段相关和简单线性相关来构建和验证两个IVIVC模型。结果线性,分段相关的预测误差(PEs)符合美国食品药品管理局(FDA)建立A级IVIVC的标准,其最大绝对内部内部PE最大值为4.6%,最大绝对内部平均PE值为3.9%,并且获得的最大绝对外部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。获得的最大绝对平均内部PE为3.9%,最大绝对外部内部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。获得的最大绝对平均内部PE为3.9%,最大绝对外部内部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。
更新日期:2019-12-19
中文翻译:

使用可挤压核心系统渗透递送技术开发和验证托法替尼缓释片的A级体外相关性。
目的确定可挤出核心系统(ECS)渗透片剂平台是否可以实现经过验证的体外A级体内相关性(IVIVC)。Tofacitinib是用于治疗类风湿关节炎的口服JAK抑制剂。方法制备11 mg托法替尼ECS片剂的快速,中度和缓释缓释制剂,以及22 mg托法替尼ECS片剂的一种制剂。托法替尼ECS片剂的体外溶解是使用USP仪器2(桨)进行的,并且从健康志愿者的1期研究中获得了体内药代动力学(PK)数据。包含5 mg速释制剂片剂,以支持托法替尼ECS PK片剂数据解卷积以获得体内吸收曲线。线性的 使用分段相关和简单线性相关来构建和验证两个IVIVC模型。结果线性,分段相关的预测误差(PEs)符合美国食品药品管理局(FDA)建立A级IVIVC的标准,其最大绝对内部内部PE最大值为4.6%,最大绝对内部平均PE值为3.9%,并且获得的最大绝对外部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。获得的最大绝对平均内部PE为3.9%,最大绝对外部内部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。获得的最大绝对平均内部PE为3.9%,最大绝对外部内部PE为8.4%。结论这项研究表明,托法替尼ECS渗透片剂平台可以达到A级IVIVC,类似于其他渗透递送系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号