当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How mechanical forces can modulate the metal affinity and selectivity of metal binding sites in proteins.
Metallomics ( IF 2.9 ) Pub Date : 2020-01-07 , DOI: 10.1039/c9mt00283a Todor Dudev 1 , Luis Manuel Frutos , Obis Castaño
Metallomics ( IF 2.9 ) Pub Date : 2020-01-07 , DOI: 10.1039/c9mt00283a Todor Dudev 1 , Luis Manuel Frutos , Obis Castaño
Affiliation
|
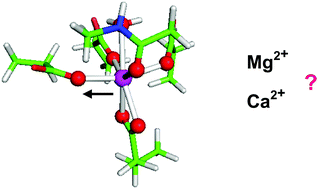
Mechanical forces play a key role in essential biological processes including cell growth, division, deformation, adhesion, migration and intra-cell interactions. The effect of mechanical forces in modulating the structure and properties of metal-occupied protein binding sites has not been fully understood. Here, by employing a combination of density functional theory (DFT) calculations and polarizable continuum model (PCM) computations applied on model metal-loaded EF-hand binding sites, we shed light on the intimate mechanism of the Mg2+/Ca2+ competition impacted by the application of mechanical stimuli. Applying mechanical force with a specific directionality and magnitude may shift the balance between the competing metal cations in favor of a given contestant depending on the composition and strength of the coordinative bonds and robustness of the metal binding site. Furthermore, the calculations help to determine the range of mechanical rupture forces typical for these structures: these range from 0.4 to 1.5 nN depending on the nature of the metal and amino acid residue. This positions the strength of the Mg2+-O and Ca2+-O coordinative bonds between that of typical covalent and hydrogen bonds. The bonds between the metal cation and the charged amino acid residue rupture at higher forces (∼1.2-1.5 nN) relative to those of their metal-noncharged counterparts which dissociate at ∼0.2-0.4 nN.
中文翻译:

机械力如何调节蛋白质中金属结合位点的金属亲和力和选择性。
机械力在必不可少的生物学过程中起关键作用,包括细胞生长,分裂,变形,粘附,迁移和细胞内相互作用。机械力在调节金属占据的蛋白质结合位点的结构和性质方面的作用尚未完全了解。在这里,通过将密度泛函理论(DFT)计算和可极化连续体模型(PCM)计算应用于模型金属负载的EF-手结合位点的组合,我们阐明了Mg2 + / Ca2 +竞争受分子动力学影响的密切机制。机械刺激的应用。施加具有特定方向性和大小的机械力可能会改变竞争性金属阳离子之间的平衡,从而有利于给定竞争者,具体取决于配位键的组成和强度以及金属结合位点的坚固性。此外,计算有助于确定这些结构的典型机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这将Mg2 + -O和Ca2 + -O配位键的强度置于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。计算有助于确定这些结构典型的机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这将Mg2 + -O和Ca2 + -O配位键的强度置于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。计算有助于确定这些结构典型的机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这使Mg2 + -O和Ca2 + -O配位键的强度介于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。
更新日期:2020-03-26
中文翻译:

机械力如何调节蛋白质中金属结合位点的金属亲和力和选择性。
机械力在必不可少的生物学过程中起关键作用,包括细胞生长,分裂,变形,粘附,迁移和细胞内相互作用。机械力在调节金属占据的蛋白质结合位点的结构和性质方面的作用尚未完全了解。在这里,通过将密度泛函理论(DFT)计算和可极化连续体模型(PCM)计算应用于模型金属负载的EF-手结合位点的组合,我们阐明了Mg2 + / Ca2 +竞争受分子动力学影响的密切机制。机械刺激的应用。施加具有特定方向性和大小的机械力可能会改变竞争性金属阳离子之间的平衡,从而有利于给定竞争者,具体取决于配位键的组成和强度以及金属结合位点的坚固性。此外,计算有助于确定这些结构的典型机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这将Mg2 + -O和Ca2 + -O配位键的强度置于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。计算有助于确定这些结构典型的机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这将Mg2 + -O和Ca2 + -O配位键的强度置于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。计算有助于确定这些结构典型的机械断裂力范围:取决于金属和氨基酸残基的性质,其范围为0.4到1.5 nN。这使Mg2 + -O和Ca2 + -O配位键的强度介于典型的共价键和氢键之间。相对于在约0.2-0.4 nN处解离的不带电荷的金属阳离子,金属阳离子和带电氨基酸残基之间的键在较高的作用力(约1.2-1.5 nN)下破裂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号