当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of the heavy metal binding properties of periplasmic metal uptake protein CLas-ZnuA2.
Metallomics ( IF 3.4 ) Pub Date : 2019-12-19 , DOI: 10.1039/c9mt00200f Pranav Kumar 1 , Vikram Dalal 1 , Nidhi Sharma 1 , Sunil Kokane 2 , Dilip Kumar Ghosh 2 , Pravindra Kumar 1 , Ashwani Kumar Sharma 1
Metallomics ( IF 3.4 ) Pub Date : 2019-12-19 , DOI: 10.1039/c9mt00200f Pranav Kumar 1 , Vikram Dalal 1 , Nidhi Sharma 1 , Sunil Kokane 2 , Dilip Kumar Ghosh 2 , Pravindra Kumar 1 , Ashwani Kumar Sharma 1
Affiliation
|
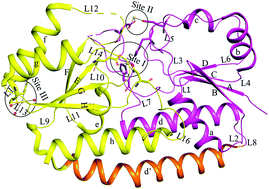
Candidatus Liberibacter asiaticus (CLas), a phloem-limited unculturable Gram-negative bacterium, causes citrus greening disease. The proteome analysis of CLas showed the presence of a heavy metal permease and Co/Zn/Cd cation exporter system. However, there is no designated metal uptake protein specific for the heavy metal permease in CLas. One of the metal uptake proteins, designated as CLas-ZnuA2, in our previous studies, showed a lower metal-binding affinity for Mn2+ and Zn2+ and was postulated to bind and transport metals rather non-specifically. The present study focused on the characterization of the heavy metal binding properties of CLas-ZnuA2 using SPR, CD, DSC and crystallographic studies. The crystal structure analysis of Cd2+ bound CLas-ZnuA2 showed octahedral geometry for Cd2+ binding as compared to a non-preferred square-pyramidal geometry for Mn2+ and Zn2+ binding in earlier reported crystal structures. In SPR analysis, the binding affinities of 4.7 × 10-6 M, 7.2 × 10-6 M, 5.3 × 10-5 M and 4.3 × 10-5 M for Hg2+, Cd2+, Ba2+ and Co2+ respectively were higher as compared to earlier reported values for Mn2+ and Zn2+. Likewise, CD and DSC analysis showed relatively higher thermal stability for CLas-ZnuA2 on heavy metal binding. Taken together with the expression of the permease and exporter system for heavy metals, our results indicate that CLas-ZnuA2 may be involved in sequestering and transport of various transition divalent metals in environmentally stressed conditions.
中文翻译:

周质金属摄取蛋白CLas-ZnuA2的重金属结合特性的表征。
韧皮部有限的无法培养的革兰氏阴性细菌亚洲假丝酵母(CLas)引起柑橘绿化病。CLas的蛋白质组分析表明存在重金属通透酶和Co / Zn / Cd阳离子输出系统。但是,在CLA中,没有针对重金属渗透酶的特异性金属摄取蛋白。在我们以前的研究中,一种金属吸收蛋白称为CLas-ZnuA2,显示出对Mn2 +和Zn2 +较低的金属结合亲和力,并被认为可以非特异性地结合和转运金属。本研究的重点是利用SPR,CD,DSC和晶体学研究表征CLas-ZnuA2的重金属结合特性。Cd2 +结合的CLas-ZnuA2的晶体结构分析显示,与Cd2 +结合的八面体几何形状相比,早期报道的晶体结构中Mn2 +和Zn2 +结合的非优选方金字塔形几何形状。在SPR分析中,Hg2 +,Cd2 +,Ba2 +和Co2 +的结合亲和力分别为4.7×10-6 M,7.2×10-6 M,5.3×10-5 M和4.3×10-5 M报告了Mn2 +和Zn2 +的值。同样,CD和DSC分析显示CLas-ZnuA2在重金属结合上具有相对较高的热稳定性。结合渗透酶和重金属出口系统的表达,我们的结果表明,CLas-ZnuA2可能在环境压力条件下与各种过渡二价金属的螯合和运输有关。
更新日期:2020-02-27
中文翻译:

周质金属摄取蛋白CLas-ZnuA2的重金属结合特性的表征。
韧皮部有限的无法培养的革兰氏阴性细菌亚洲假丝酵母(CLas)引起柑橘绿化病。CLas的蛋白质组分析表明存在重金属通透酶和Co / Zn / Cd阳离子输出系统。但是,在CLA中,没有针对重金属渗透酶的特异性金属摄取蛋白。在我们以前的研究中,一种金属吸收蛋白称为CLas-ZnuA2,显示出对Mn2 +和Zn2 +较低的金属结合亲和力,并被认为可以非特异性地结合和转运金属。本研究的重点是利用SPR,CD,DSC和晶体学研究表征CLas-ZnuA2的重金属结合特性。Cd2 +结合的CLas-ZnuA2的晶体结构分析显示,与Cd2 +结合的八面体几何形状相比,早期报道的晶体结构中Mn2 +和Zn2 +结合的非优选方金字塔形几何形状。在SPR分析中,Hg2 +,Cd2 +,Ba2 +和Co2 +的结合亲和力分别为4.7×10-6 M,7.2×10-6 M,5.3×10-5 M和4.3×10-5 M报告了Mn2 +和Zn2 +的值。同样,CD和DSC分析显示CLas-ZnuA2在重金属结合上具有相对较高的热稳定性。结合渗透酶和重金属出口系统的表达,我们的结果表明,CLas-ZnuA2可能在环境压力条件下与各种过渡二价金属的螯合和运输有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号