当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Chloro-2-nitroaniline Solubility in Several Pure Solvents: Determination, Modeling, and Solvent Effect Analysis
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jced.9b00919 Jian Wang 1 , Xin Yuan 1 , Jean-Noël Jaubert 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jced.9b00919 Jian Wang 1 , Xin Yuan 1 , Jean-Noël Jaubert 2
Affiliation
|
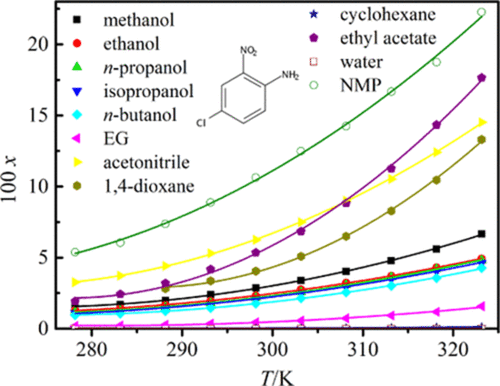
The solubility of 4-chloro-2-nitroaniline in several neat solvents of ethylene glycol (EG), N-methyl pyrrolidone (NMP), methanol, ethanol, n-propanol, isopropanol, n-butanol, cyclohexane, acetonitrile, 1,4-dioxane, ethyl acetate, and water was attained via a shake-flask method from 278.15 to 323.15 K under the pressure of approximately 101.2 kPa. Within the examined temperature range, the mole fraction solubility of 4-chloro-2-nitroaniline increased with the rising temperature. The magnitude of solubility obeyed the sequence from high to low as: NMP > (ethyl acetate, acetonitrile) > 1,4-dioxane > methanol > ethanol > n-propanol > isopropanol > n-butanol > EG > cyclohexane > water. No polymorphic transformation or solvate formation took place in these neat solvents. The molecule interactions of solvent–solvent and solvent–solute were examined through a method of linear solvation energy relationship. The Wilson model, nonrandom two-liquid model, modified Apelblat equation, and λh equation were applied to mathematically describe the 4-chloro-2-nitroaniline solubility. The obtained maximum values of root-mean-square and relative average deviations were 31.83 × 10–4 and 3.79%, respectively, which showed that the modified Apelblat equation correlated better with the solubility data than the models. Also, the mixing property was resulted from the determined solubility data.
中文翻译:

4-氯-2-硝基苯胺在几种纯溶剂中的溶解度:测定,建模和溶剂效果分析
4-氯-2-硝基苯胺在乙二醇(EG),N-甲基吡咯烷酮(NMP),甲醇,乙醇,正丙醇,异丙醇,正丁醇,环己烷,乙腈,1,4的几种纯溶剂中的溶解度通过摇瓶法在约101.2kPa的压力下从278.15至323.15K获得-二恶烷,乙酸乙酯和水。在检查的温度范围内,4-氯-2-硝基苯胺的摩尔分数溶解度随温度升高而增加。溶解度的大小遵循从高到低的顺序:NMP>(乙酸乙酯,乙腈)> 1,4-二恶烷>甲醇>乙醇>正丙醇>异丙醇> n-丁醇> EG>环己烷>水。在这些纯溶剂中没有发生多晶型转化或溶剂化物的形成。溶剂-溶剂和溶剂-溶质之间的分子相互作用通过线性溶剂化能量关系的方法进行了研究。威尔逊模型,非随机双液体模型,修正Apelblat方程,和λ ħ方程式被应用到数学上描述了4-氯-2-硝基苯胺的溶解度。所获得的均方根最大值和相对平均偏差分别为31.83×10 –4和3.79%,这表明改进的Apelblat方程与溶解度数据的相关性高于模型。同样,混合性能是由确定的溶解度数据得出的。
更新日期:2019-12-19
中文翻译:

4-氯-2-硝基苯胺在几种纯溶剂中的溶解度:测定,建模和溶剂效果分析
4-氯-2-硝基苯胺在乙二醇(EG),N-甲基吡咯烷酮(NMP),甲醇,乙醇,正丙醇,异丙醇,正丁醇,环己烷,乙腈,1,4的几种纯溶剂中的溶解度通过摇瓶法在约101.2kPa的压力下从278.15至323.15K获得-二恶烷,乙酸乙酯和水。在检查的温度范围内,4-氯-2-硝基苯胺的摩尔分数溶解度随温度升高而增加。溶解度的大小遵循从高到低的顺序:NMP>(乙酸乙酯,乙腈)> 1,4-二恶烷>甲醇>乙醇>正丙醇>异丙醇> n-丁醇> EG>环己烷>水。在这些纯溶剂中没有发生多晶型转化或溶剂化物的形成。溶剂-溶剂和溶剂-溶质之间的分子相互作用通过线性溶剂化能量关系的方法进行了研究。威尔逊模型,非随机双液体模型,修正Apelblat方程,和λ ħ方程式被应用到数学上描述了4-氯-2-硝基苯胺的溶解度。所获得的均方根最大值和相对平均偏差分别为31.83×10 –4和3.79%,这表明改进的Apelblat方程与溶解度数据的相关性高于模型。同样,混合性能是由确定的溶解度数据得出的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号