当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.freeradbiomed.2019.12.018 Brandon Griess 1 , Shakeel Mir 1 , Kaustubh Datta 1 , Melissa Teoh-Fitzgerald 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.freeradbiomed.2019.12.018 Brandon Griess 1 , Shakeel Mir 1 , Kaustubh Datta 1 , Melissa Teoh-Fitzgerald 1
Affiliation
|
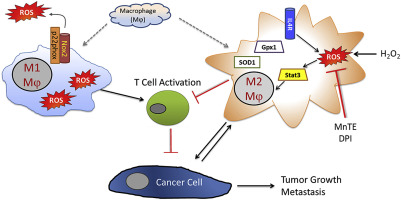
Tumor associated macrophages (TAM) enhance the aggressiveness of breast cancer via promoting cancer cell growth, metastasis, and suppression of the patient's immune system. These TAMs are polarized in breast cancer with features more closely resembling the pro-tumorigenic and immunosuppressive M2 type rather than the anti-tumor and pro-inflammatory M1 type. The goal of our study was to examine primary human monocyte-derived M1 and M2 macrophages for key redox differences and determine sensitivities of these macrophages to the redox-active drug, MnTE-2-PyP5+. This compound reduced levels of M2 markers and inhibited their ability to promote cancer cell growth and suppress T cell activation. The surface levels of the T cell suppressing molecule, PD-L2, were reduced by MnTE-2-PyP5+ in a dose-dependent manner. This study also examined key differences in ROS generation and scavenging between M1 and M2 macrophages. Our results indicate that M2 macrophages have lower levels of reactive oxygen species (ROS) and lower production of extracellular hydrogen peroxide compared to the M1 macrophages. These differences are due in part to reduced expression levels of pro-oxidants, Nox2, Nox5, and the non-enzymatic members of the Nox complex, p22phox and p47phox, as well as higher levels of antioxidant enzymes, Cu/ZnSOD, Gpx1, and catalase. More importantly, we found that despite having lower ROS levels, M2 macrophages require ROS for proper polarization, as addition of hydrogen peroxide increased M2 markers. These TAM-like macrophages are also more sensitive to the ROS modulator and a pan-Nox inhibitor. Both MnTE-2-PyP5+ and DPI inhibited expression levels of M2 marker genes. We have further shown that this inhibition was partly mediated through a decrease in Stat3 activation during IL4-induced M2 polarization. Overall, this study reveals key redox differences between M1 and M2 primary human macrophages and that redox-active drugs can be used to inhibit the pro-tumor and immunosuppressive phenotype of TAM-like M2 macrophages. This study also provides rationale for combining MnTE-2-PyP5+ with immunotherapies.
中文翻译:

清除活性氧物种通过抑制 Stat3 选择性地抑制 M2 巨噬细胞极化及其部分促肿瘤发生功能。
肿瘤相关巨噬细胞 (TAM) 通过促进癌细胞生长、转移和抑制患者的免疫系统来增强乳腺癌的侵袭性。这些 TAM 在乳腺癌中呈两极分化,其特征更类似于促肿瘤发生和免疫抑制的 M2 型,而不是抗肿瘤和促炎性 M1 型。我们研究的目的是检查原代人单核细胞衍生的 M1 和 M2 巨噬细胞的关键氧化还原差异,并确定这些巨噬细胞对氧化还原活性药物 MnTE-2-PyP5+ 的敏感性。这种化合物降低了 M2 标记物的水平,并抑制了它们促进癌细胞生长和抑制 T 细胞活化的能力。T 细胞抑制分子 PD-L2 的表面水平被 MnTE-2-PyP5+ 以剂量依赖的方式降低。该研究还检查了 M1 和 M2 巨噬细胞之间 ROS 生成和清除的主要差异。我们的结果表明,与 M1 巨噬细胞相比,M2 巨噬细胞具有较低水平的活性氧 (ROS) 和较低的细胞外过氧化氢产量。这些差异部分是由于促氧化剂 Nox2、Nox5 和 Nox 复合物的非酶成员 p22phox 和 p47phox 的表达水平降低,以及抗氧化酶 Cu/ZnSOD、Gpx1 和过氧化氢酶。更重要的是,我们发现尽管 ROS 水平较低,但 M2 巨噬细胞需要 ROS 才能正确极化,因为添加过氧化氢会增加 M2 标记物。这些 TAM 样巨噬细胞也对 ROS 调节剂和泛 Nox 抑制剂更敏感。MnTE-2-PyP5+ 和 DPI 均抑制 M2 标记基因的表达水平。我们进一步表明,这种抑制作用部分是通过 IL4 诱导的 M2 极化过程中 Stat3 激活的减少来介导的。总的来说,这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。
更新日期:2019-12-19
中文翻译:

清除活性氧物种通过抑制 Stat3 选择性地抑制 M2 巨噬细胞极化及其部分促肿瘤发生功能。
肿瘤相关巨噬细胞 (TAM) 通过促进癌细胞生长、转移和抑制患者的免疫系统来增强乳腺癌的侵袭性。这些 TAM 在乳腺癌中呈两极分化,其特征更类似于促肿瘤发生和免疫抑制的 M2 型,而不是抗肿瘤和促炎性 M1 型。我们研究的目的是检查原代人单核细胞衍生的 M1 和 M2 巨噬细胞的关键氧化还原差异,并确定这些巨噬细胞对氧化还原活性药物 MnTE-2-PyP5+ 的敏感性。这种化合物降低了 M2 标记物的水平,并抑制了它们促进癌细胞生长和抑制 T 细胞活化的能力。T 细胞抑制分子 PD-L2 的表面水平被 MnTE-2-PyP5+ 以剂量依赖的方式降低。该研究还检查了 M1 和 M2 巨噬细胞之间 ROS 生成和清除的主要差异。我们的结果表明,与 M1 巨噬细胞相比,M2 巨噬细胞具有较低水平的活性氧 (ROS) 和较低的细胞外过氧化氢产量。这些差异部分是由于促氧化剂 Nox2、Nox5 和 Nox 复合物的非酶成员 p22phox 和 p47phox 的表达水平降低,以及抗氧化酶 Cu/ZnSOD、Gpx1 和过氧化氢酶。更重要的是,我们发现尽管 ROS 水平较低,但 M2 巨噬细胞需要 ROS 才能正确极化,因为添加过氧化氢会增加 M2 标记物。这些 TAM 样巨噬细胞也对 ROS 调节剂和泛 Nox 抑制剂更敏感。MnTE-2-PyP5+ 和 DPI 均抑制 M2 标记基因的表达水平。我们进一步表明,这种抑制作用部分是通过 IL4 诱导的 M2 极化过程中 Stat3 激活的减少来介导的。总的来说,这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。这项研究揭示了 M1 和 M2 原代人巨噬细胞之间的关键氧化还原差异,并且氧化还原活性药物可用于抑制 TAM 样 M2 巨噬细胞的促肿瘤和免疫抑制表型。该研究还提供了将 MnTE-2-PyP5+ 与免疫疗法相结合的基本原理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号